RBMS2 mediates SLC7A11 transcription-translation to regulate ferroptosis in colorectal cancer
IF 8.2
2区 生物学
Q1 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
Abstract
The incidence of colorectal cancer (CRC) increases yearly. Ferroptosis, a form of regulated cell death, has gained extensive attention in cancer research. RNA-binding motif single-stranded interacting protein 2 (RBMS2) has been implicated in various cancers, but its role in CRC and its involvement with ferroptosis remains poorly understood. This study explores the involvement of RBMS2 in CRC development and its potential as a therapeutic target. Functional assays, including CCK-8, colony formation, Transwell migration, invasion assays, and EMT-related gene determination, were conducted to evaluate the effects of RBMS2 overexpression and knockdown. Ferroptosis, apoptosis, and autophagy were assessed using specific inhibitors, ferroptosis inducers, and apoptosis and proliferation detection. The interaction between RBMS2 and SLC7A11 was explored during the ferroptosis process. In vivo experiments involved xenograft models in nude mice to observe tumor growth, EMT, and metastasis. Overexpression of RBMS2 inhibited CRC cell proliferation, migration, and epithelial-mesenchymal transition (EMT). Furthermore, RBMS2 promoted ferroptosis by downregulating SLC7A11. Mechanistic studies revealed that RBMS2 destabilizes SLC7A11 at the mRNA level. In vivo, RBMS2 overexpression significantly suppressed tumor growth and lung/liver metastasis. Our findings indicate that RBMS2 inhibits CRC progression by promoting ferroptosis and regulating SLC7A11 mRNA stability. Targeting the RBMS2-SLC7A11 axis could provide a novel therapeutic strategy for CRC.
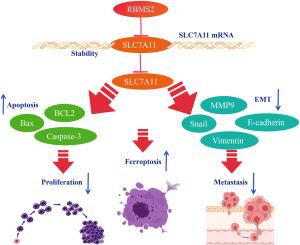
RBMS2介导SLC7A11转录-翻译调控结直肠癌铁下垂
结直肠癌(CRC)的发病率逐年上升。铁下垂是一种受调控的细胞死亡形式,在癌症研究中得到了广泛的关注。rna结合基序单链相互作用蛋白2 (RBMS2)与多种癌症有关,但其在结直肠癌中的作用及其与铁ptosis的关系尚不清楚。本研究探讨了RBMS2在结直肠癌发展中的作用及其作为治疗靶点的潜力。功能分析包括CCK-8、菌落形成、Transwell迁移、侵袭试验和emt相关基因测定,以评估RBMS2过表达和敲低的影响。通过特异性抑制剂、铁下垂诱导剂以及细胞凋亡和增殖检测来评估铁下垂、细胞凋亡和自噬。研究了RBMS2与SLC7A11在铁下垂过程中的相互作用。体内实验采用裸鼠异种移植模型来观察肿瘤生长、EMT和转移。RBMS2过表达抑制结直肠癌细胞增殖、迁移和上皮-间质转化(EMT)。此外,RBMS2通过下调SLC7A11促进铁下垂。机制研究表明,RBMS2在mRNA水平上破坏SLC7A11的稳定性。在体内,RBMS2过表达可显著抑制肿瘤生长和肺/肝转移。我们的研究结果表明,RBMS2通过促进铁下垂和调节SLC7A11 mRNA的稳定性来抑制CRC的进展。靶向RBMS2-SLC7A11轴可能为结直肠癌提供一种新的治疗策略。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Free Radical Biology and Medicine
医学-内分泌学与代谢
CiteScore
14.00
自引率
4.10%
发文量
850
审稿时长
22 days
期刊介绍:
Free Radical Biology and Medicine is a leading journal in the field of redox biology, which is the study of the role of reactive oxygen species (ROS) and other oxidizing agents in biological systems. The journal serves as a premier forum for publishing innovative and groundbreaking research that explores the redox biology of health and disease, covering a wide range of topics and disciplines. Free Radical Biology and Medicine also commissions Special Issues that highlight recent advances in both basic and clinical research, with a particular emphasis on the mechanisms underlying altered metabolism and redox signaling. These Special Issues aim to provide a focused platform for the latest research in the field, fostering collaboration and knowledge exchange among researchers and clinicians.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


