Development of highly potent and selective oncogenic KRASG12D PROTAC degraders
European Journal of Medicinal Chemistry Reports
Pub Date : 2025-08-30
DOI:10.1016/j.ejmcr.2025.100296
引用次数: 0
Abstract
As a key driver of tumorigenesis and cancer development, the KRASG12D mutation is ubiquitous existed in KRAS-associated malignancies, highlighting the urgent clinical need for effective KRASG12D targeting drugs. In this study, through rational design and multiple cell type-based antiproliferative evaluation, we identified a novel KRASG12D inhibitor, Y-I-1, which demonstrated remarkable anti-cancer activity. Subsequent computational analyses including molecular dynamics (MD) simulations, binding free energy calculations, umbrella sampling, and protein-ligand docking revealed its excellent binding characteristics, rationalizing the observed potency. Building upon the structure of Y-I-1, we constructed proteolysis-targeting chimera (PROTAC) by conjugating it with different linker moieties and VH032. Among them, degrader Y-D-2 potently and selectively exhibited nanomolar inhibitory IC50, degradation DC50 values, and more than 95 % maximum degradation (Dmax) in KRASG12D-mutant cancer cells via ubiquitin proteasome-involving pathway, accompanied by nanomolar IC50 efficiency for phosphorylated ERK (pERK) inhibition. Mechanistically, Y-D-2 significantly induced cell apoptosis, G1 cell cycle arrest and inhibited cell migration and invasion. Notably, Y-D-2 led to significant tumor growth inhibition in the GP2D xenograft model with well-tolerated dose-schedules with favorable PK properties. This study not only provides an important theoretical basis for the optimal design of KRASG12D degraders, but also highlights its potential for the treatment of KRASG12D-driven cancers.
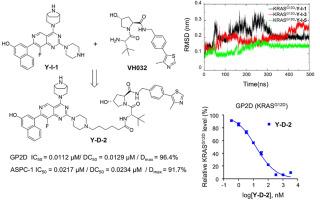
高效和选择性致癌KRASG12D PROTAC降降剂的开发
KRASG12D突变作为肿瘤发生和癌症发展的关键驱动因素,在kras相关恶性肿瘤中普遍存在,迫切需要临床有效的KRASG12D靶向药物。本研究通过合理设计和基于多细胞类型的抗增殖评价,我们鉴定出一种新的KRASG12D抑制剂Y-I-1,它具有显著的抗癌活性。随后的计算分析包括分子动力学(MD)模拟、结合自由能计算、保护伞取样和蛋白质配体对接,揭示了其良好的结合特性,使所观察到的效力更加合理。在Y-I-1结构的基础上,我们将其与不同的连接体片段和VH032偶联,构建了蛋白水解靶向嵌合体(PROTAC)。其中,降解剂Y-D-2在krasg12d突变的癌细胞中,通过泛素蛋白酶体参与途径,有效且选择性地表现出纳米摩尔抑制IC50、降解DC50值和超过95%的最大降解(Dmax),同时具有纳米摩尔抑制磷酸化ERK (pERK)的IC50效率。在机制上,Y-D-2显著诱导细胞凋亡、G1细胞周期阻滞、抑制细胞迁移和侵袭。值得注意的是,Y-D-2在GP2D异种移植模型中具有显著的肿瘤生长抑制作用,具有良好的耐受性和良好的PK特性。本研究不仅为KRASG12D降解物的优化设计提供了重要的理论依据,同时也凸显了KRASG12D驱动型癌症的治疗潜力。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


