Design, Ssynthesis and Ddocking study of 1,2,3-triazole incorporated benzoxazole-oxazole derivatives and evaluation as potential anticancer agents
IF 2.2
4区 医学
Q3 CHEMISTRY, MEDICINAL
引用次数: 0
Abstract
The study reports the design and synthesis of a new library of triazole in-corporated benzoxazole oxazole compounds (11a–j) and their in vitro cytotoxic activity evaluation. The structural reliability of the synthesized compounds was confirmed by 1H NMR, 13C NMR, and mass spectral data. We have selected ER-α and CDK2 proteins for molecular docking study of the active compounds to examine their binding interactions. We have also screened the preliminary cytotoxic activity of compounds 11a–j against four human cancer cell lines: MCF-7, A549, Colo-205, and A2780 by the MTT assay with etoposide, a well-known chemotherapy drug, as a control. All the molecules have shown strong binding interactions with A binding affinity of −8.9 to −9.4 kcal/mol with the proteins ER-α and CDK2 using CB-Dock2 online server. In our findings all the compounds demonstrated selective activity whereas four of the synthesized compounds, namely 11a, 11b, 11c, and 11d, have exhibited greater cytotoxic activity against the cancer cells with IC50 ranging from 0.18 to 3.67 μM. Drug likeliness and ADME studies infer that the compounds can be frameworks for the development of anticancer medication.
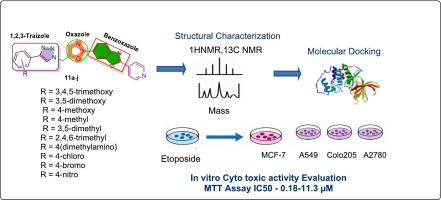
1,2,3-三唑类含苯并恶唑-恶唑衍生物的设计、合成与对接研究及潜在抗癌药物的评价
本研究报道了三唑类含苯并恶唑类新化合物(11a-j)的设计合成及其体外细胞毒活性评价。通过1H NMR、13C NMR和质谱数据证实了所合成化合物的结构可靠性。我们选择ER-α和CDK2蛋白进行活性化合物的分子对接研究,以检查它们的结合相互作用。我们还通过MTT试验筛选了化合物11a-j对四种人类癌细胞系MCF-7、A549、Colo-205和A2780的初步细胞毒活性,并以依托oposide(一种著名的化疗药物)为对照。所有分子与ER-α和CDK2蛋白的结合亲和力为−8.9 ~−9.4 kcal/mol。在我们的研究中,所有化合物都表现出选择性活性,其中4个合成的化合物11a、11b、11c和11d对癌细胞具有较强的细胞毒活性,IC50范围为0.18 ~ 3.67 μM。药物可能性和ADME研究推断,这些化合物可以作为开发抗癌药物的框架。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
5.70
自引率
3.70%
发文量
463
审稿时长
27 days
期刊介绍:
Bioorganic & Medicinal Chemistry Letters presents preliminary experimental or theoretical research results of outstanding significance and timeliness on all aspects of science at the interface of chemistry and biology and on major advances in drug design and development. The journal publishes articles in the form of communications reporting experimental or theoretical results of special interest, and strives to provide maximum dissemination to a large, international audience.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


