Heart saver: Comprehensive investigation of (redox-) proteomic and thiol metabolite changes induced by Cana-, Dapa-, Empagliflozin treatment in 2D and 3D heart cell models reveals increased mitochondrial activity and glutathione redox defense and involvement of redox signaling
IF 5.1
2区 医学
Q1 MEDICINE, RESEARCH & EXPERIMENTAL
引用次数: 0
Abstract
Aims
Antidiabetic drugs, sodium-glucose co-transporter-2 inhibitors (SGLT-2i), have demonstrated heart-saving properties independently of the diabetes status of a patient. We aimed to discover SGLT-2i-specific cardiac targets.
Materials and methods
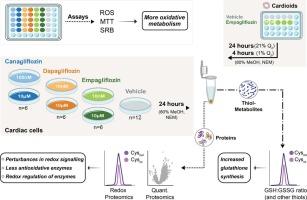
Two cardiac cell lines (AC16 and HCM) were treated with low-end therapeutic and 100- or 1000-fold dose of cana-, dapa and empagliflozin to investigate their influence on the (redox) proteome and thiol metabolome. Furthermore, we mimicked reperfusion injury (RI) on stem-cell derived cardioids to examine if and how SGLT-2i help to cope with RI.
Key findings
We show that gliflozins increase glutathione synthesis and trigger autophagy already at low drug concentration, visible through increase in ATG13. On the (redox) proteome level, several potential targets could be identified: 10 proteins affected by low concentration across all three drugs (including TXN and NAPRT) in HCM cells and 16 downregulated proteins shared between all high drug treatments in HCM cells and cardioids. Among the latter were GSR, PRDX2, LAMTOR5 and the catalytic subunit of PP2A. On the redox proteome level, we found among others, PKM, PPP1CA, PRDX5 and MDH2 with redox affected cysteine sites in both HCM and AC16 cells, and additionally, PKM, MDH2 and PPP2CA in both HCM and cardioids.
Significance
Our data suggest that gliflozin treatments affect the cells' capacity to buffer redox stress by increasing glutathione and altering redox state of key cysteine residues of proteins involved in the cellular defense against oxidative stress.
心脏拯救者:在2D和3D心脏细胞模型中,对Cana-、Dapa-、恩格列净治疗诱导的(氧化还原)蛋白质组学和硫醇代谢物变化的综合研究显示,线粒体活性和谷胱甘肽氧化还原防御以及氧化还原信号的参与增加
抗糖尿病药物钠-葡萄糖共转运蛋白-2抑制剂(SGLT-2i)已被证明具有独立于患者糖尿病状态的心脏保护特性。我们的目标是发现sglt -2i特异性心脏靶点。材料与方法用100倍或1000倍剂量的cana-、dapa和恩格列净对2株心脏细胞株AC16和HCM进行低剂量治疗,研究它们对(氧化还原)蛋白质组和硫醇代谢组的影响。此外,我们在干细胞来源的类心脏上模拟再灌注损伤(RI),以检查SGLT-2i是否以及如何帮助应对RI。我们发现格列净增加谷胱甘肽合成并在低药物浓度下触发自噬,通过ATG13的增加可见。在(氧化还原)蛋白质组水平上,可以确定几个潜在的靶点:HCM细胞中10个蛋白受所有三种药物(包括TXN和NAPRT)的低浓度影响,16个蛋白在HCM细胞和类心细胞中所有高药物治疗中共享。后者包括GSR、PRDX2、LAMTOR5和PP2A的催化亚基。在氧化还原蛋白质组水平上,我们发现PKM、PPP1CA、PRDX5和MDH2在HCM和AC16细胞中都有氧化还原影响半胱氨酸位点,此外,PKM、MDH2和PPP2CA在HCM和类心细胞中都有氧化还原影响。我们的数据表明格列净处理通过增加谷胱甘肽和改变参与细胞防御氧化应激的关键半胱氨酸残基的氧化还原状态来影响细胞缓冲氧化还原应激的能力。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Life sciences
医学-药学
CiteScore
12.20
自引率
1.60%
发文量
841
审稿时长
6 months
期刊介绍:
Life Sciences is an international journal publishing articles that emphasize the molecular, cellular, and functional basis of therapy. The journal emphasizes the understanding of mechanism that is relevant to all aspects of human disease and translation to patients. All articles are rigorously reviewed.
The Journal favors publication of full-length papers where modern scientific technologies are used to explain molecular, cellular and physiological mechanisms. Articles that merely report observations are rarely accepted. Recommendations from the Declaration of Helsinki or NIH guidelines for care and use of laboratory animals must be adhered to. Articles should be written at a level accessible to readers who are non-specialists in the topic of the article themselves, but who are interested in the research. The Journal welcomes reviews on topics of wide interest to investigators in the life sciences. We particularly encourage submission of brief, focused reviews containing high-quality artwork and require the use of mechanistic summary diagrams.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


