Redox reactivities of membrane-bound amyloid-β-Cu complexes and their targeting by metallothionein-3
IF 8.2
2区 生物学
Q1 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
Abstract
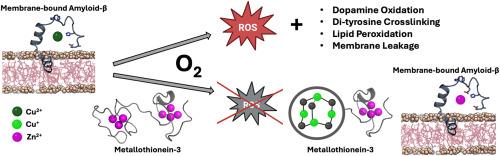
Alzheimer's disease (AD) is characterized by the accumulation of amyloid-β peptide (Aβ1-40/42) in the central nervous system (CNS). Copper coordination to Aβ triggers Aβ1-40/42 aggregation and promotes the catalytic generation of reactive oxygen species (ROS). Due to its amphiphilic nature, Aβ1-40/42 can interact with cell membranes and compromise their integrity. In this work, we characterized the insertion of Aβ1-42 into an artificial lipid bilayer system mimicking cell membranes and demonstrate that the Aβ1-42-lipid interaction does not prevent the Cu2+ coordination to Aβ1-42. We performed a comparative analysis of the redox reactivities of membrane-bound Aβ1-42 (memAβ1-42-Cu2+) with soluble Aβ1-42-Cu2+ establishing that membrane insertion leads to memAβ1-42-Cu2+ complexes featuring an enhanced detrimental catechol oxidase activity towards the neurotransmitter dopamine. Moreover, memAβ1-42-Cu2+ efficiently catalyzes Aβ di-tyrosine crosslinking and hydroxyl radical production in the presence of ascorbate. In addition, we establish that memAβ1-42-Cu2+ redox reactivity catalyzes polyunsaturated fatty acids (PUFAs) lipid peroxidation, leading to the generation of malondialdehyde (MDA) toxic end-product. This reactivity compromises the structural integrity of the lipid bilayers resulting in membrane leakage.
Metallothioneins (MTs) are cysteine-rich metalloproteins central to neuronal and astrocytic metal homeostasis. MTs bind d10 metals (Cu+ and Zn2+) forming two metal thiolate clusters in their structure. In the CNS, the metallothionein-3 (MT-3) isoform possess a neuroprotective role, but it is downregulated in AD patients. MT-3 controls aberrant protein-Cu2+ interactions and redox reactivities of amyloidogenic protein-Cu2+ complexes, including soluble Aβ1-40. In this work, we unravel that the detrimental memAβ1-42-Cu2+ redox reactivities can also be efficiently silenced by MT-3 via metal swap reactions, by scavenging and reducing Cu2+ to Cu+ in its β-domain using thiolates as electron source, forming the redox-inert Cu+4Zn2+4MT-3 species. Consequently, MT-3 efficiently prevents lipid peroxidation and protects membrane structural integrity. New strategies targeting membrane-bound Aβ1-42-Cu2+ complexes as key players in AD etiology could be envisioned.
膜结合淀粉样蛋白-β-Cu复合物的氧化还原活性及其金属硫蛋白-3的靶向性
阿尔茨海默病(AD)的特点是淀粉样蛋白-β肽(a -β 1-40/42)在中枢神经系统(CNS)积累。铜与Aβ配位触发Aβ1-40/42聚集,促进活性氧(ROS)的催化生成。由于其两亲性,a - β1-40/42可以与细胞膜相互作用并破坏其完整性。在这项工作中,我们描述了Aβ1-42插入到模拟细胞膜的人工脂质双分子层系统中,并证明了Aβ1-42与脂质相互作用不会阻止Cu2+与Aβ1-42的配位。我们对膜结合的a - β1-42(膜β1-42- cu2 +)与可溶性的a - β1-42- cu2 +的氧化还原反应进行了比较分析,确定膜插入导致膜β1-42- cu2 +复合物具有增强的有害儿茶酚氧化酶对神经递质多巴胺的活性。此外,在抗坏血酸存在的情况下,膜β1-42- cu2 +有效地催化Aβ二酪氨酸交联和羟基自由基的产生。此外,我们确定了膜β1-42- cu2 +氧化还原反应性催化多不饱和脂肪酸(PUFAs)脂质过氧化,导致产生丙二醛(MDA)毒性终产物。这种反应性破坏了脂质双分子层的结构完整性,导致膜渗漏。金属硫蛋白(MTs)是一种富含半胱氨酸的金属蛋白,对神经元和星形细胞的金属稳态至关重要。MTs结合d10金属(Cu+和Zn2+)在其结构中形成两种金属硫酯簇。在中枢神经系统中,金属硫蛋白-3 (MT-3)异构体具有神经保护作用,但在AD患者中下调。MT-3控制异常的蛋白- cu2 +相互作用和淀粉样蛋白- cu2 +复合物的氧化还原反应,包括可溶性Aβ1-40。在这项工作中,我们揭示了MT-3也可以通过金属交换反应有效地沉默有害的膜β1-42-Cu2+氧化还原反应,通过硫代酸盐作为电子源,清除Cu2+并将其还原为Cu+,形成氧化还原惰性Cu+4Zn2+4MT-3。因此,MT-3有效地防止脂质过氧化和保护膜结构的完整性。可以设想针对膜结合的a - β1-42- cu2 +复合物在AD病因学中的关键作用的新策略。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Free Radical Biology and Medicine
医学-内分泌学与代谢
CiteScore
14.00
自引率
4.10%
发文量
850
审稿时长
22 days
期刊介绍:
Free Radical Biology and Medicine is a leading journal in the field of redox biology, which is the study of the role of reactive oxygen species (ROS) and other oxidizing agents in biological systems. The journal serves as a premier forum for publishing innovative and groundbreaking research that explores the redox biology of health and disease, covering a wide range of topics and disciplines. Free Radical Biology and Medicine also commissions Special Issues that highlight recent advances in both basic and clinical research, with a particular emphasis on the mechanisms underlying altered metabolism and redox signaling. These Special Issues aim to provide a focused platform for the latest research in the field, fostering collaboration and knowledge exchange among researchers and clinicians.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


