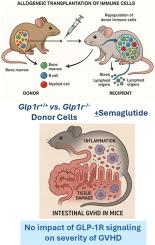
GLP-1R signaling does not modify the severity of experimental graft versus host disease
IF 6.6
2区 医学
Q1 ENDOCRINOLOGY & METABOLISM
引用次数: 0
Abstract
Objective
Glucagon-like peptide-1 (GLP-1) reduces systemic and gut inflammation. Here we assessed whether gain or loss of GLP-1 receptor (GLP-1R) signaling modifies the extent of gut injury and inflammation in experimental murine acute graft vs. host disease (aGvHD).
Methods
Allogeneic hematopoietic cell transplantation (HCT) was performed using bone marrow and splenocytes from BALB/c donors to induce aGvHD in C57BL/6 recipients or vice versa. Chimerism was determined by flow cytometry analysis of immune cell compartments. Inflammation was assessed by histological scoring of gut mucosal damage and by measuring circulating cytokine levels. qPCR was used to quantify gene expression in small intestine immune cells and tissues. The gut microbiome was assessed by 16S rRNA sequencing.
Results
Allogeneic chimerism was greater than 90% in peripheral blood and in the gut epithelial compartment. Levels of Glp1r mRNA transcripts were induced in the ileum of both vehicle- and semaglutide-treated allogeneic mice, reflecting that allogeneic T cells homing to the gut express a functional GLP-1R. Nevertheless, semaglutide did not attenuate the severity of systemic cytokine induction, gut injury or inflammation, or the extent of aGvHD in the gut mucosa. Loss of GLP-1R signaling in donor cells had limited effects on overall microbial diversity during acute GvHD, and semaglutide-treated mice exhibited modest changes in proportions of microbial species.
Conclusions
Although gut T cells express a functional GLP-1R, GLP-1R signaling has no meaningful impact on systemic or intestinal inflammation or microbiota composition in mice with experimental aGvHD, highlighting that the anti-inflammatory actions of GLP-1 medicines are highly context-dependent.
GLP-1R信号不能改变实验性移植物抗宿主病的严重程度
目的胰高血糖素样肽-1 (GLP-1)减轻全身和肠道炎症。在这里,我们评估了GLP-1受体(GLP-1R)信号的获得或丢失是否会改变实验性小鼠急性移植物抗宿主病(aGvHD)中肠道损伤和炎症的程度。方法利用BALB/c供者的骨髓和脾细胞进行sallogeneic hematopoietic cell transplantation (HCT)诱导C57BL/6受体的aGvHD,反之亦然。通过流式细胞术分析免疫细胞区室的嵌合性。通过肠黏膜损伤的组织学评分和循环细胞因子水平来评估炎症。采用qPCR定量检测小肠免疫细胞和组织中基因表达。通过16S rRNA测序评估肠道微生物组。结果外周血和肠上皮腔内同种异体嵌合率大于90%。同种异体小鼠的回肠中Glp1r mRNA转录水平均被诱导,这反映了归归肠道的同种异体T细胞表达功能性GLP-1R。然而,semaglutide并没有减轻全身细胞因子诱导、肠道损伤或炎症的严重程度,也没有减轻肠道黏膜aGvHD的程度。在急性GvHD期间,供体细胞中GLP-1R信号的丢失对总体微生物多样性的影响有限,semaglutide处理的小鼠在微生物种类的比例上表现出适度的变化。结论虽然肠道T细胞表达功能性GLP-1R,但GLP-1R信号对实验性aGvHD小鼠的全身或肠道炎症或微生物群组成没有显著影响,这表明GLP-1药物的抗炎作用高度依赖于环境。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Molecular Metabolism
ENDOCRINOLOGY & METABOLISM-
CiteScore
14.50
自引率
2.50%
发文量
219
审稿时长
43 days
期刊介绍:
Molecular Metabolism is a leading journal dedicated to sharing groundbreaking discoveries in the field of energy homeostasis and the underlying factors of metabolic disorders. These disorders include obesity, diabetes, cardiovascular disease, and cancer. Our journal focuses on publishing research driven by hypotheses and conducted to the highest standards, aiming to provide a mechanistic understanding of energy homeostasis-related behavior, physiology, and dysfunction.
We promote interdisciplinary science, covering a broad range of approaches from molecules to humans throughout the lifespan. Our goal is to contribute to transformative research in metabolism, which has the potential to revolutionize the field. By enabling progress in the prognosis, prevention, and ultimately the cure of metabolic disorders and their long-term complications, our journal seeks to better the future of health and well-being.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


