Copper-catalyzed benzo[c]isoxazoles diversification: P(O)−H bonds functionalization via ring-opening aromatization
IF 2.1
3区 化学
Q3 CHEMISTRY, INORGANIC & NUCLEAR
引用次数: 0
Abstract
The copper-catalyzed phosphorylation of benzo[c]isoxazoles with P(O)−H bonds via the in-situ ring-opening/aromatization reaction has been disclosed. This protocol is simple and convenient; a broad range of benzo[c]isoxazoles and P(O)−H compounds (e.g., H-phosphonates, H-phosphinates, and H-phosphine oxides) are well tolerated in this transformation, delivering the desired formyl-functionalized phosphoramides with good to excellent yields. In combination with step-by-step control experiments, a plausible reaction mechanism is proposed. This finding may have potential application in the synthesis of functionalized phosphoramides.
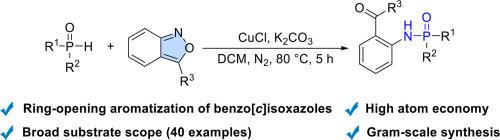
铜催化苯并[c]异恶唑多样化:开环芳构化P(O)−H键功能化
通过原位开环/芳构化反应,揭示了铜催化P(O)−H键苯并[c]异恶唑的磷酸化。该协议简单方便;广泛的苯并[c]异恶唑和P(O)−H化合物(例如,H-膦酸盐,H-膦酸盐和H-膦氧化物)在该转化中具有良好的耐受性,以良好的收率提供所需的甲酰功能化磷酰胺。结合分步控制实验,提出了一种合理的反应机理。这一发现在合成功能化磷酰胺方面具有潜在的应用价值。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Organometallic Chemistry
化学-无机化学与核化学
CiteScore
4.40
自引率
8.70%
发文量
221
审稿时长
36 days
期刊介绍:
The Journal of Organometallic Chemistry targets original papers dealing with theoretical aspects, structural chemistry, synthesis, physical and chemical properties (including reaction mechanisms), and practical applications of organometallic compounds.
Organometallic compounds are defined as compounds that contain metal - carbon bonds. The term metal includes all alkali and alkaline earth metals, all transition metals and the lanthanides and actinides in the Periodic Table. Metalloids including the elements in Group 13 and the heavier members of the Groups 14 - 16 are also included. The term chemistry includes syntheses, characterizations and reaction chemistry of all such compounds. Research reports based on use of organometallic complexes in bioorganometallic chemistry, medicine, material sciences, homogeneous catalysis and energy conversion are also welcome.
The scope of the journal has been enlarged to encompass important research on organometallic complexes in bioorganometallic chemistry and material sciences, and of heavier main group elements in organometallic chemistry. The journal also publishes review articles, short communications and notes.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


