By inhibiting pyroptosis to reduce neuroinflammation, PEG-bHb may prevent the development of secondary injury after traumatic brain injury
IF 4.2
2区 医学
Q1 NEUROSCIENCES
引用次数: 0
Abstract
Changes in severe pathological microenvironment of traumatic brain injury (TBI) have important implications for neurological repair, including oxidative stress and intense neuroinflammatory responses. Pyroptosis, a regulated cellular demise process characterized by membrane permeabilization, constitutes a primary contributor to post-traumatic brain injury induced neural inflammatory responses. Regulating the pyroptosis pathway may alleviate secondary brain injury. Polyethylene glycol-conjugated bovine hemoglobin (PEG-bHb) is a type of hemoglobin-based oxygen carriers (HBOCs) and is designed for oxygen delivery in transfusion therapy. PEG-bHb exhibits the capacity to bind and release oxygen, promoting oxygen supply in hypoxic tissues. In vivo studies have found that PEG-bHb can elevate regional oxygen saturation after TBI in prehospital stage, effectively inhibit pyroptosis, attenuate neuroinflammation and oxidative stress, and effectively attenuate the development of secondary brain injury. Furthermore, PEG-bHb has been demonstrated to protect the blood-brain barrier (BBB) by reducing the permeability of BBB and attenuating brain edema. PEG-bHb has been shown to enhance motor, learning, and memory abilities following TBI. Thus, PEG-bHb can act as a promising candidate for TBI treatment.
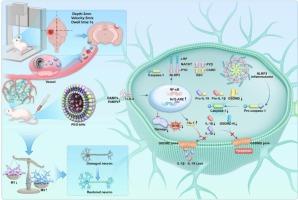
PEG-bHb可能通过抑制焦亡,减少神经炎症,从而预防创伤性脑损伤后继发性损伤的发生
创伤性脑损伤(TBI)严重病理微环境的改变对神经修复具有重要意义,包括氧化应激和强烈的神经炎症反应。焦亡是一种以膜渗透为特征的细胞死亡过程,是创伤后脑损伤诱导的神经炎症反应的主要原因。调节焦亡通路可减轻继发性脑损伤。聚乙二醇偶联牛血红蛋白(PEG-bHb)是一种基于血红蛋白的氧载体(hboc),设计用于输血治疗中的氧输送。PEG-bHb表现出结合和释放氧气的能力,促进缺氧组织的氧气供应。体内研究发现PEG-bHb可提高院前阶段TBI后局部氧饱和度,有效抑制焦亡,减轻神经炎症和氧化应激,有效减弱继发性脑损伤的发展。此外,PEG-bHb已被证明通过降低血脑屏障的通透性和减轻脑水肿来保护血脑屏障(BBB)。PEG-bHb已被证明可以增强脑外伤后的运动、学习和记忆能力。因此,PEG-bHb可以作为TBI治疗的有希望的候选药物。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Experimental Neurology
医学-神经科学
CiteScore
10.10
自引率
3.80%
发文量
258
审稿时长
42 days
期刊介绍:
Experimental Neurology, a Journal of Neuroscience Research, publishes original research in neuroscience with a particular emphasis on novel findings in neural development, regeneration, plasticity and transplantation. The journal has focused on research concerning basic mechanisms underlying neurological disorders.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


