Farrerol confers neuroprotection in spinal cord injury by regulating macrophages/microglia polarization through the JAK2/STAT3 pathway
IF 4.2
2区 医学
Q1 NEUROSCIENCES
引用次数: 0
Abstract
Background and aims
Spinal cord injury (SCI) causes secondary damage characterized by neuroinflammation and imbalanced macrophages/microglia polarization, worsening neuronal loss and functional decline. Farrerol (FAR), a natural flavonoid with anti-inflammatory properties, has not been studied for SCI treatment. This work assesses FAR's neuroprotection through macrophages/microglia polarization regulation and explores its mechanisms.
Methods
C57BL/6 mice with spinal cord injury were randomly assigned to three groups: Sham, SCI, and SCI + FAR. Motor function was evaluated using locomotor scoring, while lesion size, myelin integrity, and neuronal apoptosis were assessed via histology, immunofluorescence, and Western blot. Spinal inflammatory cytokine, macrophages/microglia activation, and polarization were analyzed by qRT-PCR, ELISA, immunofluorescence, and flow cytometry. LPS-stimulated BV2 microglia and BV2-HT22 co-cultures evaluated FAR's effects on cytokine secretion, macrophages/microglia phenotypes, and neuronal survival. Signaling mechanisms were further examined via Western blot and immunofluorescence.
Results
FAR treatment significantly enhanced motor recovery in SCI mice, evidenced by elevated Basso Mouse Scale (BMS) scores, increased inclined plane angles, improved swimming performance, and refined gait patterns. It reduced lesion area, preserved myelin integrity, and attenuated neuronal apoptosis. FAR downregulated pro-inflammatory cytokines (TNF-α, IL-1β, IL-6), suppressed macrophages/microglia hyperactivation while upregulating IL-10, and shifted M1/M2 polarization toward neuroprotective M2 phenotypes. In LPS-stimulated BV2 microglia, FAR attenuated inflammatory responses, inhibited M1 markers, enhanced M2 markers, and rescued HT22 neuronal apoptosis in co-cultures. These therapeutic effects may be mediated through suppression of JAK2/STAT3 phosphorylation.
Conclusions
FAR promotes functional recovery after spinal cord injury by modulating macrophages/microglia M1/M2 polarization through JAK2/STAT3 pathway inhibition, thereby attenuating neuroinflammation and neuronal death. These findings provide novel evidence supporting targeted immunomodulation for SCI treatment.
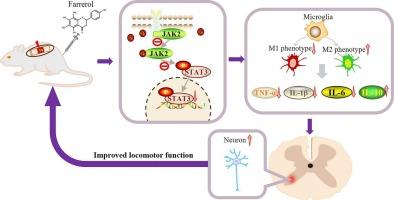
法瑞罗通过JAK2/STAT3通路调节巨噬细胞/小胶质细胞极化,从而在脊髓损伤中发挥神经保护作用
背景与目的脊髓损伤(SCI)可引起继发性损伤,其特征为神经炎症和巨噬细胞/小胶质细胞极化失衡,神经元损失加重和功能下降。法罗醇(FAR)是一种具有抗炎特性的天然类黄酮,尚未被研究用于脊髓损伤治疗。本研究通过巨噬细胞/小胶质细胞极化调节评估FAR的神经保护作用,并探讨其机制。方法将sc57bl /6脊髓损伤小鼠随机分为Sham组、SCI组和SCI + FAR组。通过运动评分评估运动功能,通过组织学、免疫荧光和Western blot评估病变大小、髓磷脂完整性和神经元凋亡。采用qRT-PCR、ELISA、免疫荧光和流式细胞术分析脊髓炎性细胞因子、巨噬细胞/小胶质细胞活化和极化情况。lps刺激的BV2小胶质细胞和BV2- ht22共培养评估FAR对细胞因子分泌、巨噬细胞/小胶质细胞表型和神经元存活的影响。通过Western blot和免疫荧光进一步检测信号机制。结果far治疗显著增强了脊髓损伤小鼠的运动恢复,表现为Basso小鼠量表(BMS)评分升高、斜面角增加、游泳表现改善和步态模式改善。减少病变面积,保持髓磷脂完整性,减轻神经元凋亡。FAR下调促炎细胞因子(TNF-α, IL-1β, IL-6),抑制巨噬细胞/小胶质细胞的过度活化,上调IL-10,使M1/M2极化向神经保护性M2表型转移。在lps刺激的BV2小胶质细胞中,FAR可以减轻炎症反应,抑制M1标记,增强M2标记,并挽救HT22神经元的凋亡。这些治疗效果可能是通过抑制JAK2/STAT3磷酸化介导的。结论far通过抑制JAK2/STAT3通路,调节巨噬细胞/小胶质细胞M1/M2极化,促进脊髓损伤后功能恢复,从而减轻神经炎症和神经元死亡。这些发现为支持靶向免疫调节治疗脊髓损伤提供了新的证据。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Experimental Neurology
医学-神经科学
CiteScore
10.10
自引率
3.80%
发文量
258
审稿时长
42 days
期刊介绍:
Experimental Neurology, a Journal of Neuroscience Research, publishes original research in neuroscience with a particular emphasis on novel findings in neural development, regeneration, plasticity and transplantation. The journal has focused on research concerning basic mechanisms underlying neurological disorders.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


