Phase 1/2 trial of encorafenib, cetuximab, and nivolumab in microsatellite stable BRAFV600E metastatic colorectal cancer
IF 44.5
1区 医学
Q1 CELL BIOLOGY
引用次数: 0
Abstract
The BRAF inhibitor encorafenib and anti-epidermal growth factor receptor (EGFR) antibody cetuximab modestly improve survival for patients with microsatellite stable (MSS) BRAFV600E metastatic colorectal cancer (mCRC), characterized by higher immune activation than MSS BRAFwild-type colorectal cancer (CRC). In this phase 1/2 study (NCT04017650) of 26 participants with MSS BRAFV600E mCRC who received encorafenib, cetuximab, and anti-PD-1 antibody nivolumab, we report an overall response rate of 50% (95% confidence interval [CI] 29–71) and median progression-free survival of 7.4 months (95% CI, 5.6–9.6). Transcriptomic profiling of pretreatment biopsies and extracellular vesicle RNA (evRNA) isolated from plasma show enrichment of non-canonical mitogen-activated protein kinase (MAPK) signaling and immune activation signatures for responders. Complement pathway activation enriches in non-responder biopsies. On serial evRNA profiling, decreased MAPK signature and increased interferon gamma response signature associate with sustained treatment benefit. MSS BRAFV600E mCRC with baseline MAPK activation and immune activation signatures may benefit from the triple combination but not with complement pathway activation.
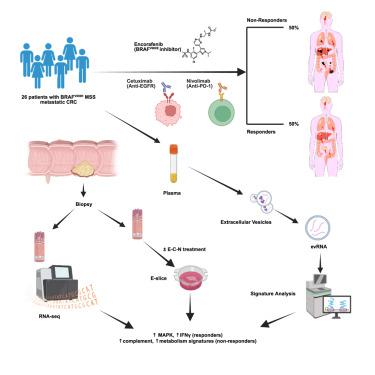
恩科非尼、西妥昔单抗和纳武单抗治疗微卫星稳定BRAFV600E转移性结直肠癌的1/2期试验
BRAF抑制剂encorafenib和抗表皮生长因子受体(EGFR)抗体西妥昔单抗可适度提高微卫星稳定型(MSS) BRAFV600E转移性结直肠癌(mCRC)患者的生存率,其特征是比MSS braf野生型结直肠癌(CRC)具有更高的免疫激活。在这项1/2期研究(NCT04017650)中,26名MSS BRAFV600E mCRC患者接受了恩科非尼、西妥昔单抗和抗pd -1抗体纳武单抗,我们报告总缓解率为50%(95%置信区间[CI] 29-71),中位无进展生存期为7.4个月(95% CI, 5.6-9.6)。预处理活检和从血浆中分离的细胞外囊泡RNA (evRNA)的转录组学分析显示,应答者的非典型有丝分裂原活化蛋白激酶(MAPK)信号和免疫激活信号富集。补体通路激活在无应答活检中丰富。在连续的evRNA分析中,MAPK信号的减少和干扰素γ反应信号的增加与持续的治疗益处相关。具有基线MAPK激活和免疫激活特征的MSS BRAFV600E mCRC可能受益于三联疗法,但不受益于补体途径激活。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Cancer Cell
医学-肿瘤学
CiteScore
55.20
自引率
1.20%
发文量
179
审稿时长
4-8 weeks
期刊介绍:
Cancer Cell is a journal that focuses on promoting major advances in cancer research and oncology. The primary criteria for considering manuscripts are as follows:
Major advances: Manuscripts should provide significant advancements in answering important questions related to naturally occurring cancers.
Translational research: The journal welcomes translational research, which involves the application of basic scientific findings to human health and clinical practice.
Clinical investigations: Cancer Cell is interested in publishing clinical investigations that contribute to establishing new paradigms in the treatment, diagnosis, or prevention of cancers.
Insights into cancer biology: The journal values clinical investigations that provide important insights into cancer biology beyond what has been revealed by preclinical studies.
Mechanism-based proof-of-principle studies: Cancer Cell encourages the publication of mechanism-based proof-of-principle clinical studies, which demonstrate the feasibility of a specific therapeutic approach or diagnostic test.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


