Fast and selective protein modification with iron-substituted polyoxometalates via a radical pathway
IF 6.4
1区 化学
Q1 CHEMISTRY, INORGANIC & NUCLEAR
引用次数: 0
Abstract
Oxidative modifications of proteins are crucial post-translational modifications that profoundly impact their structure, function, and turnover. Developing chemical methods that selectively induce oxidative protein modifications and cleavage would significantly facilitate elucidation of these oxidative processes, benefiting our understanding of disease mechanisms, identifying novel therapeutic targets, and advancing biotechnological applications. In this work, we demonstrate that all-inorganic discrete polyoxometalate (POM) clusters stabilize redox active metal centers such as Fe(III) and Mn(III) under physiological pH and temperature (pH = 7.5, 37 °C), enabling the generation of reactive oxygen species (ROS) under mild aqueous conditions. Specifically, we show that catalytic amounts of the iron-substituted POM K7[FeIII(α2-P2W17O61)(H2O)] (FeIIIWD), in the presence of ascorbate (Asc), rapidly induce selective oxidation and cleavage of hen egg-white lysozyme (HEWL) in four narrow regions of the protein sequence. The protein cleavage sites are all located near the interaction sites of MIIIWD (M = Mn or Fe) catalysts with the protein surface. In contrast, the manganese-substituted POM K7[MnIII(α2-P2W17O61)(H2O)] (MnIIIWD) shows no similar catalytic activity, pointing towards a different radical mechanism. These findings highlight the potential of well-tailored inorganic clusters to facilitate selective catalytic processes, enabling iron to target specific regions of a protein sequence without relying on coordination sites on the protein surface, while offering flexibility in reaction conditions.
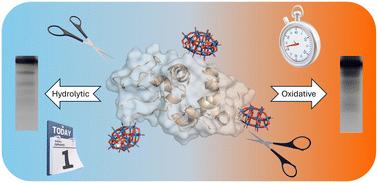
铁取代的多金属氧酸盐通过自由基途径快速和选择性地修饰蛋白质
蛋白质的氧化修饰是重要的翻译后修饰,深刻影响其结构,功能和周转。开发选择性诱导氧化蛋白修饰和切割的化学方法将极大地促进这些氧化过程的阐明,有利于我们对疾病机制的理解,确定新的治疗靶点,并推进生物技术的应用。在这项工作中,我们证明了全无机离散多金属氧酸盐(POM)簇在生理pH和温度(pH = 7.5, 37°C)下稳定氧化还原活性金属中心,如Fe(III)和Mn(III),使在温和的水条件下产生活性氧(ROS)。具体来说,我们发现在抗坏血酸(Asc)存在的情况下,铁取代的POM K7[FeIII(α2-P2W17O61)(H2O)] (feiiwd)的催化量可以在蛋白序列的四个狭窄区域快速诱导蛋清溶菌酶(HEWL)的选择性氧化和裂解。蛋白质的裂解位点均位于miiwd (M = Mn或Fe)催化剂与蛋白质表面相互作用位点附近。相比之下,锰取代的POM K7[MnIII(α2-P2W17O61)(H2O)] (MnIIIWD)没有类似的催化活性,表明其自由基机制不同。这些发现强调了精心定制的无机簇促进选择性催化过程的潜力,使铁能够靶向蛋白质序列的特定区域,而不依赖于蛋白质表面的配位位点,同时在反应条件上提供灵活性。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Inorganic Chemistry Frontiers
CHEMISTRY, INORGANIC & NUCLEAR-
CiteScore
10.40
自引率
7.10%
发文量
587
审稿时长
1.2 months
期刊介绍:
The international, high quality journal for interdisciplinary research between inorganic chemistry and related subjects
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


