Access to valuable 1,4- and 1,5-diketones through gold(i) catalysis in water: application to chemoenzymatic cascades†
IF 4.2
3区 化学
Q2 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
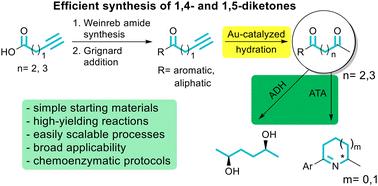
1,4- and 1,5-diketones are remarkable structures in different fields as they serve as precursors of many valuable derivatives such as heterocycles and they are also present in the skeleton of a wide variety of natural products and biologically active compounds. Herein, we propose a novel and general methodology that combines the use of both Weinreb and gold chemistries under mild conditions. Thus, starting from pent-4-ynoic or hex-5-ynoic acid, the corresponding Weinreb amides were efficiently obtained, and then after the reaction with a suitable Grignard reagent, a series of alk-4-yn-1-ones and alk-5-yn-1-ones were synthesized in high to excellent yields (74–94%). Later, these compounds were hydrated using the gold(i) catalyst JohnPhosAuCl and the additive NaBArF4 at very low loadings in an aqueous medium and at mild temperature, affording the desired dicarbonylic derivatives at high extent (92–98%). This method was also applied to various aliphatic 1,ω-diynes, which were transformed into the corresponding diketones (88–93%). Due to the mildness of this reaction, it could be combined with different biocatalysts in a one-pot sequential or concurrent approach to access a valuable tetrahydropyridine (1 g scale, 94% isolated yield, >99% ee) or a relevant diol (200 mg scale, 88% isolated yield, >99% ee, >99% de).
通过金(i)在水中催化获得有价值的1,4-和1,5-二酮:在化学酶级联中的应用†
1,4-二酮和1,5-二酮是许多有价值的衍生物(如杂环)的前体,在许多天然产物和生物活性化合物的骨架中都存在,因此在不同的领域具有重要的结构意义。在这里,我们提出了一种新的和通用的方法,结合使用Weinreb和金化学在温和的条件下。因此,从戊-4-炔酸或己-5-炔酸开始,高效地得到相应的Weinreb酰胺,然后与合适的格氏试剂反应,以高收率(74-94%)合成了一系列的烷基-4-炔-1和烷基-5-炔-1- 1。随后,用金(I)催化剂JohnPhosAuCl和添加剂NaBArF4在水介质中以非常低的负载和温和的温度水化这些化合物,得到所需的高程度(92-98%)的二羰基衍生物。该方法也适用于各种脂肪族1,ω-二炔,转化为相应的二酮(88-93%)。由于该反应的温和性,它可以与不同的生物催化剂在一锅连续或并行的方法中结合,以获得有价值的四氢吡啶(1g规模,94%分离收率,>99% ee)或相关的二醇(200mg规模,88%分离收率,>99% ee, >99% de)。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Catalysis Science & Technology
CHEMISTRY, PHYSICAL-
CiteScore
8.70
自引率
6.00%
发文量
587
审稿时长
1.5 months
期刊介绍:
A multidisciplinary journal focusing on cutting edge research across all fundamental science and technological aspects of catalysis.
Editor-in-chief: Bert Weckhuysen
Impact factor: 5.0
Time to first decision (peer reviewed only): 31 days
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


