Carbon surface chemistry drives speciation and reactivity of cationic Fe species in CO2 activation: a HERFD-XANES and valence-to-core XES study†
IF 4.2
3区 化学
Q2 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
While carbon-supported iron nanostructures are able to provide inexpensive frameworks where the dispersion of single-atom centres enables unique catalytic properties for carbon dioxide functionalization, detailed understanding of the structure of the transition metals is often prevented by the heterogeneous nature of the hosting C matrix and the variety of available sites, consequently hindering the understanding and development of CO2 reduction chemistry. Herein, we report an experimental and computational spectroscopic investigation of few-layer graphene-based samples decorated with Fe atoms immobilised at the edges and in-plane defects of the graphene layers. We find that Fe–OH bound to N-terminated edge sites or in-plane defects of the graphene layers reacts with CO2, forming bicarbonates. A similar reactivity is observed for Fe–OH bound to C-terminated edge sites, whereas Fe–OH coordinated to C-terminated in-plane defects remains unreactive towards CO2. In stark contrast, FeN4 sites in Fe–porphyrin present a direct, carbon-atom-mediated interaction with CO2. These results provide insights into the local coordination environment of iron and its role in the reactivity towards CO2 activation.
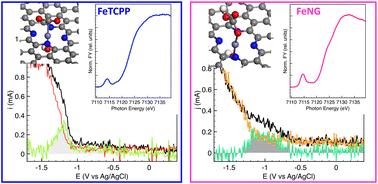
碳表面化学驱动二氧化碳活化中阳离子铁物种的形成和反应性:HERFD-XANES和价核XES研究†
虽然碳支撑的铁纳米结构能够提供廉价的框架,其中单原子中心的分散能够实现二氧化碳功能化的独特催化性能,但对过渡金属结构的详细了解往往受到宿主C基质的异质性质和各种可用位点的阻碍,从而阻碍了对二氧化碳还原化学的理解和发展。在此,我们报告了一项实验和计算光谱研究,研究了在石墨烯层的边缘和面内缺陷处固定铁原子装饰的少层石墨烯基样品。我们发现Fe-OH结合到石墨烯层的n端边缘位置或平面内缺陷与CO2反应,形成碳酸氢盐。结合到c端边缘位置的铁- oh具有类似的反应性,而配合到c端平面内缺陷的铁- oh对CO2没有反应性。与之形成鲜明对比的是,fe -卟啉中的FeN4位点与CO2存在碳原子介导的直接相互作用。这些结果为铁的局部配位环境及其在CO2活化反应性中的作用提供了见解。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Catalysis Science & Technology
CHEMISTRY, PHYSICAL-
CiteScore
8.70
自引率
6.00%
发文量
587
审稿时长
1.5 months
期刊介绍:
A multidisciplinary journal focusing on cutting edge research across all fundamental science and technological aspects of catalysis.
Editor-in-chief: Bert Weckhuysen
Impact factor: 5.0
Time to first decision (peer reviewed only): 31 days
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


