Coordinatively unsaturated Lewis acidic aluminum sites in zeolites for direct partial oxidation of methane†
IF 4.2
3区 化学
Q2 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
In this study, we show that transition metal-free aluminosilicate-type zeolites (Al-zeolites) are active and highly selective for partial oxidation of CH4 (POM) to CO at temperatures ranging from 600 to 800 °C by using O2 as an oxidizing agent. Although the POM activities of Al-zeolites depend on their topologies, CO selectivity was high at around 80% even at CH4 conversions above 20%, regardless of the topologies. CHA and Beta zeolites exhibit relatively high and stable catalytic performance over a reaction time of 6 h, while MFI zeolite was highly active at the initial period and then quickly deactivated with prolonged duration. MOR and FAU zeolites show low POM activity. The acid sites in Al-zeolites after the POM reaction were analyzed by infrared (IR) spectroscopy using CO as a molecular probe. The results illustrate that the Al-zeolites, with a greater abundance of coordinatively unsaturated Lewis acidic Al, result in higher POM activity. A mechanistic study demonstrates that the C−H bond was activated by a strong LAS derived from a coordinatively unsaturated Al atom via a four-centered transition state. Computational activation energy was revealed as 32 kcal mol−1, being close to the experimental value (34 kcal mol−1).
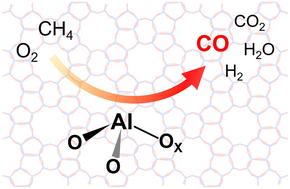
甲烷直接部分氧化沸石中配位不饱和路易斯酸铝位
在这项研究中,我们证明了过渡金属铝硅酸盐型沸石(al-沸石)在600至800℃的温度范围内,以O2作为氧化剂,对CH4 (POM)部分氧化成CO具有活性和高选择性。尽管al分子筛的POM活性取决于其拓扑结构,但无论拓扑结构如何,即使在CH4转化率超过20%时,CO选择性也高达80%左右。CHA和Beta分子筛在6 h的反应时间内表现出较高且稳定的催化性能,而MFI分子筛在反应初期表现出较高的活性,但随着反应时间的延长,催化活性迅速下降。MOR和FAU分子筛的POM活性较低。以CO为分子探针,用红外光谱分析了POM反应后铝沸石中的酸位。结果表明,配位不饱和路易斯酸Al丰度较高的Al分子筛具有较高的POM活性。机理研究表明,C−H键是由配位不饱和Al原子通过四中心过渡态产生的强LAS激活的。计算活化能为32 kcal mol−1,接近实验值(34 kcal mol−1)。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Catalysis Science & Technology
CHEMISTRY, PHYSICAL-
CiteScore
8.70
自引率
6.00%
发文量
587
审稿时长
1.5 months
期刊介绍:
A multidisciplinary journal focusing on cutting edge research across all fundamental science and technological aspects of catalysis.
Editor-in-chief: Bert Weckhuysen
Impact factor: 5.0
Time to first decision (peer reviewed only): 31 days
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


