Unlocking high selectivity and stability of a cobalt-based catalyst in the n-butanol amination reaction†
IF 4.2
3区 化学
Q2 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
Primary amines, exemplified by n-butylamine, serve as critical intermediates in the synthesis of pharmaceuticals and agrochemicals. Amination of n-butanol with ammonia over supported cobalt catalysts represents a promising synthetic route. To enhance the catalytic performance of cobalt-based amination catalysts, we reported an acid-treated strategy that allows for precise regulation of cobalt speciation. Among the evaluated supports, silicalite-1 demonstrated superior amination performance, attributed to its unique ability to enhance cobalt dispersion and suppress acid-induced side reactions. Through acid treatment, oversized Co3O4 nanoparticles are selectively removed, thereby preserving highly dispersed cobalt species. Under rigorous reaction conditions (WHSV = 2.5 h−1), the acid-treated catalyst achieved 90% selectivity toward n-butylamine, accompanied by improved stability compared to untreated counterparts. Mechanistic investigations revealed that well-dispersed metallic Co0 nanoparticles promoted selective C–N bond formation via efficient coupling, whereas larger cobalt domains facilitated dehydrogenation-driven carbon deposition pathways. This work establishes a clear structure–performance relationship for cobalt-based amination catalysts, offering a blueprint for sustainable amine production.
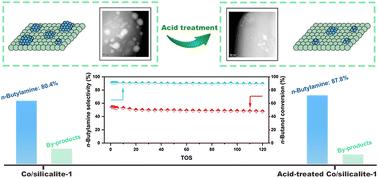
正丁醇胺化反应中钴基催化剂的高选择性和稳定性研究
伯胺,例如正丁胺,是合成药物和农用化学品的关键中间体。负载型钴催化剂上氨化正丁醇是一条很有前途的合成途径。为了提高钴基胺化催化剂的催化性能,我们报道了一种酸处理策略,可以精确调节钴的形态。在评估的支撑体中,硅石-1表现出优越的胺化性能,这归功于其独特的增强钴分散和抑制酸诱导副反应的能力。通过酸处理,超大的Co3O4纳米颗粒被选择性地去除,从而保留了高度分散的钴物种。在严格的反应条件下(WHSV = 2.5 h−1),酸处理的催化剂对正丁胺的选择性达到90%,同时与未处理的催化剂相比,稳定性有所提高。机制研究表明,分散良好的金属Co0纳米颗粒通过高效偶联促进了选择性C-N键的形成,而较大的钴畴则促进了脱氢驱动的碳沉积途径。本研究为钴基胺化催化剂建立了清晰的结构-性能关系,为可持续胺生产提供了蓝图。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Catalysis Science & Technology
CHEMISTRY, PHYSICAL-
CiteScore
8.70
自引率
6.00%
发文量
587
审稿时长
1.5 months
期刊介绍:
A multidisciplinary journal focusing on cutting edge research across all fundamental science and technological aspects of catalysis.
Editor-in-chief: Bert Weckhuysen
Impact factor: 5.0
Time to first decision (peer reviewed only): 31 days
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


