Solid micellar catalysts: exploring the stability of Ru(III) single-sites in amorphous silica†
IF 4.2
3区 化学
Q2 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
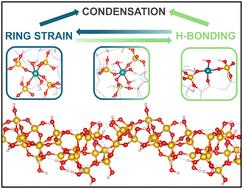
Amorphous silica plays an important role as a catalyst support due to its structural and chemical properties, though its complexity poses challenges identifying and characterizing active sites. Computational models capturing the heterogeneity of amorphous silica-supported materials are crucial in understanding these systems and their catalytic behavior. Ru(III)@MCM-41, the first example of a solid micellar catalyst, consists of Ru(III) sites and silanoxo basic sites incorporated into the walls of amorphous MCM-41 and stabilized by quaternary ammonium surfactant molecules (CTA+) in the pores. To link structure and activity, computational models were developed to elucidate the nature and activity of these Ru(III) single-sites. The amorphous silica framework offers a wide heterogeneity of sites where ruthenium can be located. To understand how site distribution affects the activity and stability of Ru(III) sites, a periodic model of an amorphous hydroxylated silica surface was used. The model includes 27 potential Si-to-Ru(III) exchange and multiple Ru(III) grafting sites, from bulk-like Si(O)4 to single and geminal silanol groups (Si(O)3(OH) and Si(O)2(OH)2, respectively). The coordination environment, ring strain, and the hydrogen bonding interactions influence the stability and activity of the Ru(III) single-sites. Incorporating Ru(III) in amorphous silica is highly favorable, with Si-to-Ru(III) exchange energies up to −248 kJ mol−1 in the presence of the CTA+ surfactant. Replacing the surfactant counterion with H+ results in a less favorable incorporation of Ru(III), with exchange energies up to −118 kJ mol−1, highlighting the surfactant's stabilizing role. Grafting of Ru(III) onto silanoxo groups is also favorable in the presence of surfactant. However, space restrictions and the limited availability of neighboring silanol groups at an optimal distance made the formation of Ru(III)(O)4 challenging, instead favoring less stable Ru(III)(O)2(OH)2 species. Our calculations suggest that Ru(III) is predominantly present as Ru(III)(O)4(H2O)2 or Ru(III)(O)3(OH)(H2O)2 at sites within large Si-membered rings near hydroxylated regions. Various Ru(III)-hydride species were calculated, with formation energies ranging from +21 to −113 kJ mol−1. Interestingly, some highly stable Ru(III) sites also show favorable hydride formation, making them both synthesizable and active.
固体胶束催化剂:探索Ru(III)在无定形二氧化硅†中单位点的稳定性
无定形二氧化硅由于其结构和化学性质而扮演着催化剂载体的重要角色,尽管其复杂性给活性位点的识别和表征带来了挑战。计算模型捕捉非晶硅支撑材料的非均质性对于理解这些系统及其催化行为至关重要。Ru(III)@MCM-41是固体胶束催化剂的第一个例子,它是由Ru(III)位点和硅氧基位点结合到无定形MCM-41的壁上,并由孔中的季铵表面活性剂分子(CTA+)稳定组成的。为了将结构和活性联系起来,开发了计算模型来阐明这些Ru(III)单位点的性质和活性。无定形二氧化硅框架提供了广泛的非均匀性的地点,其中钌可以定位。为了了解位置分布如何影响Ru(III)位置的活性和稳定性,使用了无定形羟基化二氧化硅表面的周期模型。该模型包括27个潜在的Si- Ru(III)交换和多个Ru(III)接枝位点,从大块状的Si(O)4到单硅醇基团和双硅醇基团(分别为Si(O)3(OH)和Si(O)2(OH)2)。配位环境、环应变和氢键相互作用影响Ru(III)单位点的稳定性和活性。在CTA+表面活性剂存在下,Ru(III)与Ru(III)的交换能高达−248 kJ mol−1。用H+取代表面活性剂的反离子导致Ru(III)的掺入不利,交换能高达- 118 kJ mol - 1,突出了表面活性剂的稳定作用。在表面活性剂的存在下,Ru(III)接枝到硅氧基上也是有利的。然而,空间限制和最佳距离上邻近硅醇基团的有限可用性使得Ru(III)(O)4的形成具有挑战性,反而有利于稳定性较差的Ru(III)(O)2(OH)2。我们的计算表明,Ru(III)主要以Ru(III)(O)4(H2O)2或Ru(III)(O)3(OH)(H2O)2的形式存在于靠近羟基化区域的大硅元环内。计算了不同种类的Ru(III)氢化物,生成能范围为+21 ~−113 kJ mol−1。有趣的是,一些高度稳定的Ru(III)位点也显示出有利的氢化物形成,使它们既可合成又具有活性。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Catalysis Science & Technology
CHEMISTRY, PHYSICAL-
CiteScore
8.70
自引率
6.00%
发文量
587
审稿时长
1.5 months
期刊介绍:
A multidisciplinary journal focusing on cutting edge research across all fundamental science and technological aspects of catalysis.
Editor-in-chief: Bert Weckhuysen
Impact factor: 5.0
Time to first decision (peer reviewed only): 31 days
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


