Crude levulinic acid conversion to valeric acid over La–TiO2 supported Ni catalysts: an environmentally friendly route to fuel additive synthesis†
IF 4.2
3区 化学
Q2 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
Transformation of biomass into energy or other valuable products is a sustainable route. This study encompasses the single step synthesis of valeric acid by hydrodeoxygenation of biomass derived crude levulinic acid using formic acid as a hydrogen source over different loadings of lanthanum modified TiO2-supported Ni catalysts. Modification with La resulted in a comparatively low ratio of acid sites on the catalyst surface. Formic acid adsorbed IR studies revealed that 10 wt%Ni/3 wt%La–TiO2 displayed a high proportion of surface basic sites, which were responsible for the high rate of valeric acid (∼56 × 10−8 mol gcat−1 s−1) with a stable activity up to 48 h. Pyridine and acetone adsorbed IR results indicated an increase in the La loading from 1 to 5 wt% on TiO2 and a decrease in the Lewis and Brønsted acidic sites and carbonyl interactions with the catalyst, respectively. Considering the probe (HCOOH, acetone, pyridine) adsorbed IR spectroscopic results, levulinic acid conversion and the product distribution, a plausible surface reaction mechanism has been proposed.
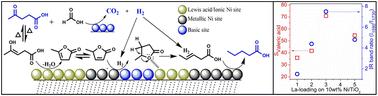
在La-TiO2负载的Ni催化剂上将粗乙酰丙酸转化为戊酸:一种环保的燃料添加剂合成途径
将生物质转化为能源或其他有价值的产品是一种可持续的途径。本研究以甲酸为氢源,在不同负载的镧修饰tio2负载的Ni催化剂上,通过对生物质衍生的粗乙酰丙酸加氢脱氧,一步合成戊酸。用La修饰后,催化剂表面的酸位比例相对较低。甲酸吸附的IR研究表明,10 wt%Ni/3 wt%La - TiO2表面碱性位点的比例很高,这是导致戊酸的高速率(~ 56 × 10−8 mol gcat−1 s−1)的原因,并且活性稳定到48 h。吡啶和丙酮吸附的IR结果表明,TiO2上的La负载从1 wt%增加到5 wt%, Lewis和Brønsted酸性位点和羰基与催化剂的相互作用分别减少。结合探针(HCOOH、丙酮、吡啶)吸附的红外光谱结果、乙酰丙酸的转化和产物的分布,提出了一种合理的表面反应机理。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Catalysis Science & Technology
CHEMISTRY, PHYSICAL-
CiteScore
8.70
自引率
6.00%
发文量
587
审稿时长
1.5 months
期刊介绍:
A multidisciplinary journal focusing on cutting edge research across all fundamental science and technological aspects of catalysis.
Editor-in-chief: Bert Weckhuysen
Impact factor: 5.0
Time to first decision (peer reviewed only): 31 days
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


