Protocatechuic aldehyde restrains NLRP3 inflammasome activation to alleviate inflammatory response in sepsis
IF 2.9
3区 医学
Q2 PHARMACOLOGY & PHARMACY
引用次数: 0
Abstract
Sepsis, a life-threatening organ dysfunction syndrome triggered by infection, is characterized by complex pathophysiology involving dysregulated inflammation, coagulation abnormalities, and mitochondrial dysfunction. Excessive activation of the NLRP3 inflammasome plays a pivotal role in sepsis progression. This study investigated the therapeutic effects and underlying mechanisms of protocatechuic aldehyde (PCA) in sepsis. Seventy-five potential PCA targets for sepsis were identified, with KEGG enrichment highlighting involvement in inflammatory and apoptotic pathways. PPI network analysis pinpointed TNF, IL-6, and IL-1β as key inflammatory targets. PCA dose-dependently suppressed IL-1β and TNF-α release in LPS/ATP-stimulated macrophages, reduced ASC speck formation and NLRP3-ASC interaction, and decreased mt-ROS production and TXNIP-NLRP3 co-localization. PCA also preserved mitochondrial network integrity by interacting with mitochondrial dynamics proteins DRP1 and MFN2, improving mitochondrial membrane potential and morphology. In LPS-induced septic mice, PCA significantly reduced serum IL-1β and TNF-α levels, improved survival rates, and downregulated NLRP3, pro-IL-1β, and cleaved-IL-1β expression in peritoneal macrophages. PCA alleviates inflammatory responses and organ damage in septic mice by inhibiting the mt-ROS/TXNIP/NLRP3 signaling axis and maintaining mitochondrial function, offering a promising natural therapeutic candidate for sepsis.
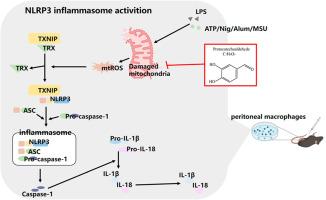
原儿茶醛抑制NLRP3炎性体激活以减轻脓毒症的炎症反应
脓毒症是一种由感染引发的危及生命的器官功能障碍综合征,其特点是复杂的病理生理,包括炎症失调、凝血异常和线粒体功能障碍。NLRP3炎性小体的过度激活在脓毒症的进展中起关键作用。本研究探讨了原儿茶醛(PCA)对脓毒症的治疗作用及其机制。鉴定出75个潜在的脓毒症PCA靶点,其中KEGG富集突出了炎症和凋亡途径的参与。PPI网络分析确定TNF, IL-6和IL-1β是关键的炎症靶点。PCA剂量依赖性地抑制LPS/ atp刺激的巨噬细胞IL-1β和TNF-α释放,减少ASC斑点形成和NLRP3-ASC相互作用,减少mt-ROS产生和TXNIP-NLRP3共定位。PCA还通过与线粒体动力学蛋白DRP1和MFN2相互作用,改善线粒体膜电位和形态,从而保持线粒体网络的完整性。在lps诱导的脓毒症小鼠中,PCA显著降低血清IL-1β和TNF-α水平,提高存活率,下调腹腔巨噬细胞中NLRP3、pro-IL-1β和cleaved-IL-1β的表达。PCA通过抑制mt-ROS/TXNIP/NLRP3信号轴和维持线粒体功能,减轻脓毒症小鼠的炎症反应和器官损伤,为脓毒症提供了一种有前景的天然治疗候选药物。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
6.20
自引率
2.90%
发文量
104
审稿时长
31 days
期刊介绍:
Journal of Pharmacological Sciences (JPS) is an international open access journal intended for the advancement of pharmacological sciences in the world. The Journal welcomes submissions in all fields of experimental and clinical pharmacology, including neuroscience, and biochemical, cellular, and molecular pharmacology for publication as Reviews, Full Papers or Short Communications. Short Communications are short research article intended to provide novel and exciting pharmacological findings. Manuscripts concerning descriptive case reports, pharmacokinetic and pharmacodynamic studies without pharmacological mechanism and dose-response determinations are not acceptable and will be rejected without peer review. The ethnopharmacological studies are also out of the scope of this journal. Furthermore, JPS does not publish work on the actions of biological extracts unknown chemical composition.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


