Lnc-THAP7-AS1 suppresses the ovarian cancer progression by targeting miR-92b-5p/fatty acid 2-hydroxylase signal axis
IF 5
2区 医学
Q2 Medicine
引用次数: 0
Abstract
Background
Ovarian cancer (OC) ranks among the most lethal gynecological malignancies. Research has highlighted long noncoding RNAs (lncRNAs) as promising novel molecular targets for cancer therapy. This study endeavors to identify novel lncRNAs, elucidate their specific roles, and unravel their regulatory mechanism in OC development.
Methods
Utilizing LncRNA Microarray technology, we screened for differentially expressed RNAs, ultimately selecting THAP7-AS1 for in-depth investigation. RNA pull-down, luciferase assays and FISH were performed to validate the interaction among THAP7-AS1, miR-92b-5p and FA2H. qRT-PCR was conducted to assess the expression of THAP7-AS1, miR-92b-5p and FA2H in OC tissues and cell lines. Cellular biological effects were examined using proliferation CCK-8 and EdU assays, chamber Transwell migration assays, and flow cytometer for apoptosis analysis. Additionally, nude mice xenograft models were established to evaluate the in vivo effect of THAP7-AS1 on tumor growth.
Results
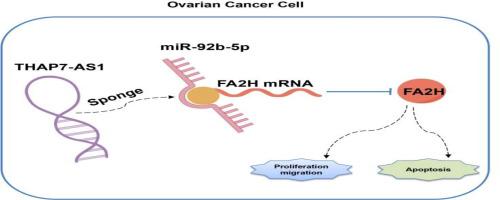
Our findings revealed a downregulation of THAP7-AS1 in OC tissues and cells. Overexpression of THAP7-AS1 inhibited cell proliferation, migration, and induced apoptosis. miR-92b-5p was identified as a sponge target of THAP7-AS1, with its expression upregulated in OC tissues and cells. Forced expression of miR-92b-5p exhibited cellular effects opposite to those of THAP7-AS1 overexpression. Additionally, FA2H was confirmed as a direct target of miR-92b-5p Silencing FA2H suppressed apoptosis, promoted proliferation and migration. In vivo experiments showed overexpression of THAP7-AS1 significantly reduced tumor volume and weight.
Conclusion
THAP7-AS1 is firstly reported as a tumor suppressor in OC by targeting the miR-92b-5p/FA2H axis. Our findings suggest that THAP7-AS1 holds potential as therapeutic target in OC treatment.
Lnc-THAP7-AS1通过靶向miR-92b-5p/脂肪酸2-羟化酶信号轴抑制卵巢癌进展
卵巢癌(OC)是最致命的妇科恶性肿瘤之一。研究强调长链非编码rna (lncRNAs)是癌症治疗中有前景的新分子靶点。本研究旨在鉴定新的lncrna,阐明其在OC发育中的具体作用,并揭示其调控机制。方法利用LncRNA微阵列技术筛选差异表达rna,最终选择THAP7-AS1进行深入研究。通过RNA下拉、荧光素酶测定和FISH来验证THAP7-AS1、miR-92b-5p和FA2H之间的相互作用。采用qRT-PCR检测THAP7-AS1、miR-92b-5p、FA2H在OC组织和细胞系中的表达情况。细胞生物学效应采用增殖CCK-8和EdU测定,室Transwell迁移测定和流式细胞仪进行凋亡分析。此外,我们还建立了裸鼠异种移植模型,以评估THAP7-AS1对肿瘤生长的体内影响。结果在OC组织和细胞中THAP7-AS1表达下调。THAP7-AS1过表达抑制细胞增殖、迁移,诱导细胞凋亡。miR-92b-5p被鉴定为THAP7-AS1的海绵靶点,在OC组织和细胞中表达上调。强迫表达miR-92b-5p表现出与THAP7-AS1过表达相反的细胞效应。此外,FA2H被证实是miR-92b-5p的直接靶点,沉默FA2H抑制细胞凋亡,促进增殖和迁移。体内实验表明,过表达THAP7-AS1可显著降低肿瘤体积和重量。结论thap7 - as1首次被报道为OC中的肿瘤抑制因子,其靶向miR-92b-5p/FA2H轴。我们的研究结果表明THAP7-AS1有潜力作为OC治疗的治疗靶点。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Translational Oncology
ONCOLOGY-
CiteScore
8.40
自引率
2.00%
发文量
314
审稿时长
54 days
期刊介绍:
Translational Oncology publishes the results of novel research investigations which bridge the laboratory and clinical settings including risk assessment, cellular and molecular characterization, prevention, detection, diagnosis and treatment of human cancers with the overall goal of improving the clinical care of oncology patients. Translational Oncology will publish laboratory studies of novel therapeutic interventions as well as clinical trials which evaluate new treatment paradigms for cancer. Peer reviewed manuscript types include Original Reports, Reviews and Editorials.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


