Chronic psilocybin administration increases sociability and alters the gut microbiome in male wild-type mice but not in a preclinical model of obsessive-compulsive disorder
IF 4.6
2区 医学
Q1 NEUROSCIENCES
引用次数: 0
Abstract
Psilocybin, a serotonergic compound that produces psychedelic effects primarily through activation of the 5-HT2A receptor, has shown promise in treating neuropsychiatric conditions, including obsessive-compulsive disorder (OCD). However, the effects of chronic psilocybin administration on gut function, microbiota, and behavioural phenotypes remain understudied. The present study investigated the effects of chronic psilocybin (0.1 and 1 mg/kg, oral gavage) on gut and behavioural measures in wild-type (WT) and SAPAP3 knockout (KO) mice, a model of OCD-like phenotypes. We present novel evidence that SAPAP3 KO mice exhibit social deficits, and that chronic psilocybin increases sociability in male WT mice. Although no therapeutic effects were observed at either dose on anxiety-, compulsive-, or depressive-like behaviour, chronic psilocybin also did not induce psychosis-like behaviours. A dose-dependent effect of psilocybin was observed on gut motility. Although chronic administration did not significantly alter overall gut microbiome diversity, reductions in Lactobacillus murinus, Lactobacillus animalis, and Alistipes dispar were observed in male WT mice, but not in KO mice or female mice. Integrative analysis revealed that a microbial cluster, comprising Lactobacillus and Alistipes species, correlated with locomotion, head-twitch response and gut motility, effectively differentiating psilocybin-treated mice from vehicle controls. This suggests a potential host-microbiome feedback mechanism regulating host serotonin signalling, linked to central and peripheral 5-HT2A receptor activation. Additionally, separate microbial clusters were associated with startle response and sociability, indicating that psilocybin may engage distinct neural pathways to mediate these behaviours. These findings highlight the importance of considering the microbiome and sex in future psychedelic research and open new avenues for exploring the microbiota-gut-brain axis as a target for future therapeutic strategies.
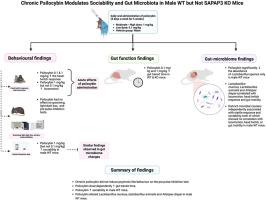
长期服用裸盖菇素增加雄性野生型小鼠的社交能力并改变肠道微生物群,但在强迫症的临床前模型中没有
裸盖菇素是一种5-羟色胺能化合物,主要通过激活5-HT2A受体产生迷幻效果,在治疗包括强迫症(OCD)在内的神经精神疾病方面显示出前景。然而,长期服用裸盖菇素对肠道功能、微生物群和行为表型的影响仍未得到充分研究。本研究研究了慢性裸盖菇素(0.1和1 mg/kg,灌胃)对野生型(WT)和SAPAP3敲除(KO)小鼠(一种强迫症样表型模型)肠道和行为指标的影响。我们提出了新的证据,SAPAP3 KO小鼠表现出社交缺陷,而慢性裸盖菇素增加了雄性WT小鼠的社交能力。尽管两种剂量的裸盖菇素对焦虑、强迫或抑郁样行为均未观察到治疗效果,但慢性裸盖菇素也不会诱发精神病样行为。裸盖菇素对肠道运动有剂量依赖性。虽然慢性给药没有显著改变整体肠道微生物群多样性,但在雄性WT小鼠中观察到鼠乳杆菌、动物乳杆菌和异丙乳杆菌的减少,而在KO小鼠和雌性小鼠中则没有。综合分析显示,由乳酸杆菌和Alistipes组成的微生物群与运动、头抽搐反应和肠道运动相关,有效地区分了裸盖菇素治疗小鼠和对照小鼠。这表明一种潜在的宿主-微生物反馈机制调节宿主血清素信号,与中枢和外周5-HT2A受体激活有关。此外,不同的微生物群与惊吓反应和社交能力有关,表明裸盖菇素可能参与不同的神经通路来调节这些行为。这些发现强调了在未来的迷幻研究中考虑微生物组和性别的重要性,并为探索微生物-肠道-脑轴作为未来治疗策略的靶点开辟了新的途径。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Neuropharmacology
医学-神经科学
CiteScore
10.00
自引率
4.30%
发文量
288
审稿时长
45 days
期刊介绍:
Neuropharmacology publishes high quality, original research and review articles within the discipline of neuroscience, especially articles with a neuropharmacological component. However, papers within any area of neuroscience will be considered. The journal does not usually accept clinical research, although preclinical neuropharmacological studies in humans may be considered. The journal only considers submissions in which the chemical structures and compositions of experimental agents are readily available in the literature or disclosed by the authors in the submitted manuscript. Only in exceptional circumstances will natural products be considered, and then only if the preparation is well defined by scientific means. Neuropharmacology publishes articles of any length (original research and reviews).
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


