Sex-specific metabolic and central effects of GLP-1–estradiol conjugate in middle-aged rats on a standard or western diet
IF 7.6
2区 医学
Q1 IMMUNOLOGY
引用次数: 0
Abstract
Middle age represents a critical window for metabolic and cognitive health, particularly in the context of rising obesity and diabetes rates. Glucagon-like peptide-1 (GLP-1)-based therapies, which regulate blood glucose and body weight, show sex-specific effects, with estradiol potentiating their metabolic benefits. However, research on GLP-1′s cognitive and neuroprotective roles has largely been conducted in males. Here, we investigated the effects of GLP-1 conjugated to estradiol (GE2) on metabolism, cognition, cytokine levels and neurogenesis in the dentate gyrus of middle-aged male and female rats fed a standard (SD) or Western (WD) diet. In both sexes, WD increased body weight and plasma leptin levels, regardless of sex. GE2 treatment led to weight loss, enhanced cued and contextual fear memory, reduced cytokine levels in the hippocampus in SD rats, and increased neurogenesis in the dorsal dentate gyrus (DG), regardless of sex. Sex-specific differences were observed in fat distribution, glucose regulation, central cytokine levels, and neuroplasticity after WD and GE2 treatment. In females only, GE2 reduced visceral (gonadal) fat, reduced cytokines in the dorsal hippocampus, and improved basal blood glucose in response to a WD. In males only, GE2 restored neurogenesis in the DG after WD exposure, and reduced cytokine levels in the amygdala. These findings suggest that although WD increased body weight and GE2 improved associative learning in both sexes, both WD and GE2 had differential affects on metabolic hormones, insulin regulation, cytokine levels and neuroplasticity. Our findings underscore the importance of sex-specific approaches in metabolic and neuroprotective therapeutics in middle age.
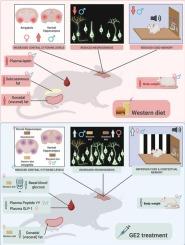
glp -1 -雌二醇缀合物对标准饮食或西式饮食的中年大鼠的性别特异性代谢和中枢效应
中年是新陈代谢和认知健康的关键时期,尤其是在肥胖和糖尿病发病率不断上升的背景下。以胰高血糖素样肽-1 (GLP-1)为基础的治疗,调节血糖和体重,显示出性别特异性的效果,雌二醇增强了它们的代谢益处。然而,关于GLP-1的认知和神经保护作用的研究主要在男性中进行。在这里,我们研究了GLP-1与雌二醇(GE2)结合对喂食标准(SD)或西方(WD)饮食的中年雄性和雌性大鼠齿状回代谢、认知、细胞因子水平和神经发生的影响。无论男女,WD都会增加体重和血浆瘦素水平。GE2治疗导致SD大鼠体重减轻,线索和情境恐惧记忆增强,海马细胞因子水平降低,背齿状回(DG)神经发生增加,与性别无关。WD和GE2治疗后,小鼠在脂肪分布、葡萄糖调节、中枢细胞因子水平和神经可塑性方面存在性别差异。仅在女性中,GE2减少了内脏(性腺)脂肪,减少了海马背侧的细胞因子,并改善了WD后的基础血糖。仅在雄性小鼠中,GE2可恢复WD暴露后DG中的神经发生,并降低杏仁核中的细胞因子水平。这些发现表明,尽管WD增加了体重,GE2改善了两性的联想学习,但WD和GE2对代谢激素、胰岛素调节、细胞因子水平和神经可塑性的影响存在差异。我们的研究结果强调了性别特异性方法在中年代谢和神经保护治疗中的重要性。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
29.60
自引率
2.00%
发文量
290
审稿时长
28 days
期刊介绍:
Established in 1987, Brain, Behavior, and Immunity proudly serves as the official journal of the Psychoneuroimmunology Research Society (PNIRS). This pioneering journal is dedicated to publishing peer-reviewed basic, experimental, and clinical studies that explore the intricate interactions among behavioral, neural, endocrine, and immune systems in both humans and animals.
As an international and interdisciplinary platform, Brain, Behavior, and Immunity focuses on original research spanning neuroscience, immunology, integrative physiology, behavioral biology, psychiatry, psychology, and clinical medicine. The journal is inclusive of research conducted at various levels, including molecular, cellular, social, and whole organism perspectives. With a commitment to efficiency, the journal facilitates online submission and review, ensuring timely publication of experimental results. Manuscripts typically undergo peer review and are returned to authors within 30 days of submission. It's worth noting that Brain, Behavior, and Immunity, published eight times a year, does not impose submission fees or page charges, fostering an open and accessible platform for scientific discourse.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


