Breakdown of blood–brain barrier, astrocytic endfeet swelling, and abnormal behaviors by blockade of TROY signaling in TROY-Fc transgenic mice
IF 7.6
2区 医学
Q1 IMMUNOLOGY
引用次数: 0
Abstract
An orphan receptor of tumor necrosis factor receptor superfamily (TNFRSF), TROY (TNFRSF19), forms the receptor complex with Nogo-66 receptor/LINGO-1 and DR6 in neurons and cerebral endothelial cells, respectively. Although TROY is expressed in astrocytes of the brain under normal conditions, its function is still unknown. Here, we demonstrated that TROY was strongly expressed in astrocytes rather than in neurons and endothelial cells in the adult mouse brain under normal conditions. Recombinant soluble form of the extracellular domain (ECD) of mouse TROY (sTROY) bound to megalencephalic leukoencephalopathy with subcortical cysts 1 (Mlc1)-positive astrocytes, endfeet of which were surrounding large vessels, in the adult mouse brain. These findings suggest the presence of an unidentified ligand that directly binds to TROY in such astrocytes. To antagonize TROY ligand(s) in vivo, we generated transgenic (Tg) mice overexpressing a chimeric protein consisting of the ECD of mouse TROY and the Fc domain of human immunoglobulin G1 (TROY-Fc). Adult TROY-Fc Tg mice showed the enhanced permeability of the blood–brain barrier (BBB), which is consistent with TROY-deficient mice, and swollen astrocytic endfeet. The expressions of tight junction proteins (claudin-5, claudin-12, occludin, and zonula occludens-1) and src-suppressed C-kinase substrate (SSeCKS) were reduced in adult Tg mice, whereas aquaporin-4 (AQP4) expression was increased. In postnatal Tg mice, the expressions of vascular endothelial growth factor and AQP4 were increased in TROY-Fc Tg mice, whereas angiopoietin-1 and SSeCKS were reduced. There was no significant change in the expression of endogenous TROY in postnatal and adult Tg mice. Behavioral tests revealed that TROY-Fc Tg mice exhibited a reduction in exploratory motivation and an enhancement of responses to stress. Thus, the signaling mediated by unidentified TROY ligand(s)-TROY interaction may be essential for proper neuronal function through the maintenance of intact BBB.
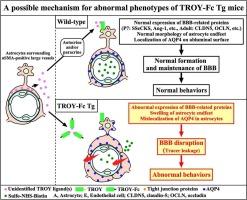
TROY- fc转基因小鼠血脑屏障破坏、星形细胞性终足肿胀及行为异常
肿瘤坏死因子受体超家族(TNFRSF)的孤儿受体TROY (TNFRSF19)分别在神经元和脑内皮细胞中与Nogo-66受体/LINGO-1和DR6形成受体复合物。TROY在正常情况下在脑星形胶质细胞中表达,但其功能尚不清楚。在这里,我们证明TROY在正常条件下在成年小鼠大脑的星形胶质细胞中而不是在神经元和内皮细胞中强烈表达。小鼠TROY (sTROY)的细胞外结构域(ECD)的重组可溶性形式与成年小鼠大脑中伴有皮层下囊肿1 (Mlc1)阳性的巨脑白质脑病星形细胞结合,其终足围绕着大血管。这些发现表明,在这些星形胶质细胞中存在一种未识别的配体直接与TROY结合。为了在体内拮抗TROY配体,我们产生了转基因(Tg)小鼠,过表达由小鼠TROY的ECD和人免疫球蛋白G1 (TROY-Fc)的Fc结构域组成的嵌合蛋白。成年TROY-Fc Tg小鼠表现出血脑屏障(BBB)通透性增强,这与troy缺乏小鼠一致,并且星形细胞终足肿胀。成年Tg小鼠紧密连接蛋白(claudin-5、claudin-12、occludin和zonula occluden -1)和src抑制的c激酶底物(SSeCKS)的表达减少,而水通道蛋白-4 (AQP4)的表达增加。在出生后Tg小鼠中,TROY-Fc Tg小鼠血管内皮生长因子和AQP4的表达增加,而血管生成素-1和SSeCKS的表达减少。出生后和成年Tg小鼠内源性TROY的表达无明显变化。行为测试显示,TROY-Fc Tg小鼠表现出探索动机的减少和对压力的反应的增强。因此,未知的TROY配体-TROY相互作用介导的信号传导可能是通过维持血脑屏障完整来维持正常神经元功能所必需的。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
29.60
自引率
2.00%
发文量
290
审稿时长
28 days
期刊介绍:
Established in 1987, Brain, Behavior, and Immunity proudly serves as the official journal of the Psychoneuroimmunology Research Society (PNIRS). This pioneering journal is dedicated to publishing peer-reviewed basic, experimental, and clinical studies that explore the intricate interactions among behavioral, neural, endocrine, and immune systems in both humans and animals.
As an international and interdisciplinary platform, Brain, Behavior, and Immunity focuses on original research spanning neuroscience, immunology, integrative physiology, behavioral biology, psychiatry, psychology, and clinical medicine. The journal is inclusive of research conducted at various levels, including molecular, cellular, social, and whole organism perspectives. With a commitment to efficiency, the journal facilitates online submission and review, ensuring timely publication of experimental results. Manuscripts typically undergo peer review and are returned to authors within 30 days of submission. It's worth noting that Brain, Behavior, and Immunity, published eight times a year, does not impose submission fees or page charges, fostering an open and accessible platform for scientific discourse.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


