Lymphoma accelerates T cell and tissue aging
IF 44.5
1区 医学
Q1 CELL BIOLOGY
引用次数: 0
Abstract
The combined effects of aging and cancer on immune cells were investigated in young versus aged mice harboring B cell lymphoma, and in T cells from young and aged B cell lymphoma patients. These analyses revealed that lymphoma alone is sufficient to trigger transcriptional, epigenetic, and phenotypic alterations in young T cells that manifest in aged T cells. In contrast, aged T cells are largely resistant to lymphoma-induced changes. Pathway analyses revealed open chromatin regions and genes controlling iron homeostasis are induced by both lymphoma and aging, and lymphoma-experienced and aged T cells have increased iron pools and are resistant to ferroptosis. Furthermore, both aged and lymphoma-experienced T cells have defects in proteostasis. B cell lymphoma also accelerates aging of other tissues, as evidenced by elevated expression of
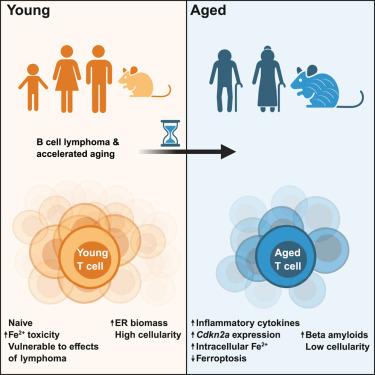
淋巴瘤加速T细胞和组织老化
研究人员在携带B细胞淋巴瘤的年轻和老年小鼠以及来自年轻和老年B细胞淋巴瘤患者的T细胞中研究了衰老和癌症对免疫细胞的综合影响。这些分析表明,淋巴瘤本身足以触发年轻T细胞的转录、表观遗传和表型改变,这些改变在老年T细胞中表现出来。相反,衰老的T细胞对淋巴瘤引起的变化有很大的抵抗力。通路分析显示,开放的染色质区域和控制铁稳态的基因都是由淋巴瘤和衰老诱导的,淋巴瘤经历和衰老的T细胞有增加的铁池,并对铁凋亡具有抗性。此外,衰老和淋巴瘤经历的T细胞都有蛋白质平衡缺陷。B细胞淋巴瘤还会加速其他组织的衰老,Cdkn2a和Tnfa的表达升高就是证据。最后,一些淋巴瘤引起的衰老表型是可逆的,而另一些是固定的,这表明有机会改善一些癌症相关的衰老合并症。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Cancer Cell
医学-肿瘤学
CiteScore
55.20
自引率
1.20%
发文量
179
审稿时长
4-8 weeks
期刊介绍:
Cancer Cell is a journal that focuses on promoting major advances in cancer research and oncology. The primary criteria for considering manuscripts are as follows:
Major advances: Manuscripts should provide significant advancements in answering important questions related to naturally occurring cancers.
Translational research: The journal welcomes translational research, which involves the application of basic scientific findings to human health and clinical practice.
Clinical investigations: Cancer Cell is interested in publishing clinical investigations that contribute to establishing new paradigms in the treatment, diagnosis, or prevention of cancers.
Insights into cancer biology: The journal values clinical investigations that provide important insights into cancer biology beyond what has been revealed by preclinical studies.
Mechanism-based proof-of-principle studies: Cancer Cell encourages the publication of mechanism-based proof-of-principle clinical studies, which demonstrate the feasibility of a specific therapeutic approach or diagnostic test.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


