Genome-wide CRISPR screens identify critical targets to enhance CAR-NK cell antitumor potency
IF 44.5
1区 医学
Q1 CELL BIOLOGY
引用次数: 0
Abstract
Adoptive cell therapy using engineered natural killer (NK) cells is a promising approach for cancer treatment, with targeted gene editing offering the potential to further enhance their therapeutic efficacy. However, the spectrum of actionable genetic targets to overcome tumor and microenvironment-mediated immunosuppression remains largely unexplored. We performed multiple genome-wide CRISPR screens in primary human NK cells and identified critical checkpoints regulating resistance to immunosuppressive pressures. Ablation of
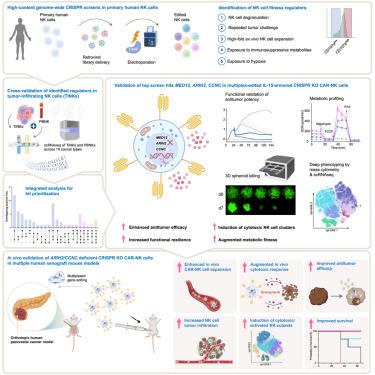
全基因组CRISPR筛选鉴定增强CAR-NK细胞抗肿瘤效力的关键靶点
使用工程化自然杀伤(NK)细胞的过继细胞治疗是一种很有前途的癌症治疗方法,有针对性的基因编辑提供了进一步提高其治疗效果的潜力。然而,克服肿瘤和微环境介导的免疫抑制的可操作的遗传靶点的谱仍然很大程度上未被探索。我们在原代人NK细胞中进行了多个全基因组CRISPR筛选,并确定了调节免疫抑制压力抗性的关键检查点。在体外和体内实验中,消融MED12、ARIH2和CCNC可显著提高NK细胞对多种难治性人类癌症的抗肿瘤活性。CRISPR编辑增强了先天和car介导的NK细胞功能,与增强的代谢适应性、促炎细胞因子的分泌增加和细胞毒性NK细胞亚群的扩增有关。通过在NK细胞中进行高含量的全基因组CRISPR筛选,本研究揭示了NK细胞功能的关键调控因子,为设计下一代NK细胞治疗方法提供了宝贵的资源,提高了NK细胞的抗癌效果。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Cancer Cell
医学-肿瘤学
CiteScore
55.20
自引率
1.20%
发文量
179
审稿时长
4-8 weeks
期刊介绍:
Cancer Cell is a journal that focuses on promoting major advances in cancer research and oncology. The primary criteria for considering manuscripts are as follows:
Major advances: Manuscripts should provide significant advancements in answering important questions related to naturally occurring cancers.
Translational research: The journal welcomes translational research, which involves the application of basic scientific findings to human health and clinical practice.
Clinical investigations: Cancer Cell is interested in publishing clinical investigations that contribute to establishing new paradigms in the treatment, diagnosis, or prevention of cancers.
Insights into cancer biology: The journal values clinical investigations that provide important insights into cancer biology beyond what has been revealed by preclinical studies.
Mechanism-based proof-of-principle studies: Cancer Cell encourages the publication of mechanism-based proof-of-principle clinical studies, which demonstrate the feasibility of a specific therapeutic approach or diagnostic test.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


