Targeting MondoA–TXNIP restores antitumour immunity in lactic-acid-induced immunosuppressive microenvironment
IF 20.8
1区 医学
Q1 ENDOCRINOLOGY & METABOLISM
引用次数: 0
Abstract
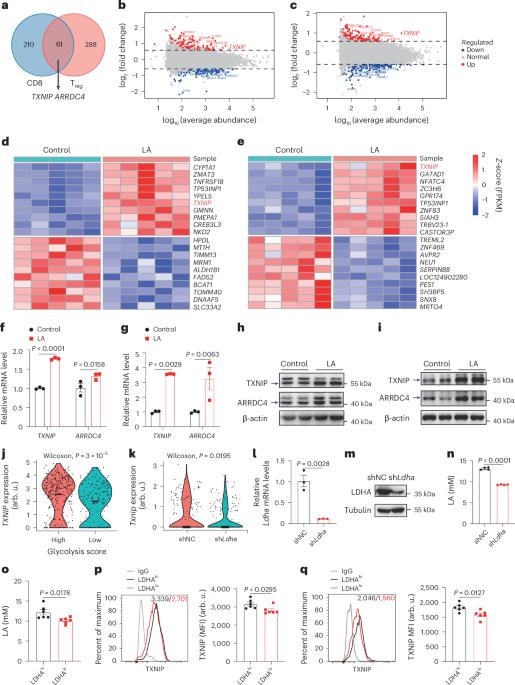
In the tumour microenvironment, accumulated lactic acid (LA) promotes tumour immune evasion by facilitating regulatory T cell (Treg) immunosuppressive function and restraining CD8+ T cell cytotoxicity, but the underlying mechanism remains elusive. Here we report that transcriptional factor MondoA-induced thioredoxin interacting protein (TXNIP) transcription is a common feature of both Treg and CD8+ T cells in response to lactic acid. In contrast to reduction in immunosuppressive capacity in MondoA-deficient Treg cells, loss of MondoA enhanced CD8+ T cell cytotoxic function in the lactic-acid-induced immunosuppressive microenvironment, by restoring glucose uptake and glycolysis. Mechanistically, lactic acid relied on sentrin/SUMO-specific protease 1 (SENP1) to stimulate the MondoA–TXNIP axis, which impaired TCR/CD28-signal-induced CD8+ T cell activation. Importantly, targeting the MondoA–TXNIP axis potentiated antitumour immunity in multiple cancer types and synergized with anti-PD-1 therapy to promote effective T cell responses in colorectal cancer. Our results demonstrate that the MondoA–TXNIP axis is a promising therapeutic target for improving cancer immunotherapy. Xu et al. identify the MondoA–TXNIP signalling axis as a regulator of antitumour immune surveillance in response to lactic acid in the tumour microenvironment.
靶向MondoA-TXNIP在乳酸诱导的免疫抑制微环境中恢复抗肿瘤免疫
在肿瘤微环境中,积累的乳酸(LA)通过促进调节性T细胞(Treg)免疫抑制功能和抑制CD8+ T细胞的细胞毒性来促进肿瘤免疫逃避,但其潜在机制尚不明确。在这里,我们报道了转录因子mondoa诱导的硫氧还蛋白相互作用蛋白(TXNIP)转录是Treg和CD8+ T细胞对乳酸反应的共同特征。与MondoA缺陷Treg细胞免疫抑制能力降低相反,在乳酸诱导的免疫抑制微环境中,MondoA缺失通过恢复葡萄糖摄取和糖酵解,增强了CD8+ T细胞的细胞毒性功能。在机制上,乳酸依赖于sentrin/ sumo特异性蛋白酶1 (SENP1)刺激MondoA-TXNIP轴,从而损害TCR/ cd28信号诱导的CD8+ T细胞活化。重要的是,靶向MondoA-TXNIP轴增强了多种癌症类型的抗肿瘤免疫,并与抗pd -1治疗协同促进结直肠癌的有效T细胞应答。我们的研究结果表明,MondoA-TXNIP轴是一个有希望改善癌症免疫治疗的治疗靶点。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Nature metabolism
ENDOCRINOLOGY & METABOLISM-
CiteScore
27.50
自引率
2.40%
发文量
170
期刊介绍:
Nature Metabolism is a peer-reviewed scientific journal that covers a broad range of topics in metabolism research. It aims to advance the understanding of metabolic and homeostatic processes at a cellular and physiological level. The journal publishes research from various fields, including fundamental cell biology, basic biomedical and translational research, and integrative physiology. It focuses on how cellular metabolism affects cellular function, the physiology and homeostasis of organs and tissues, and the regulation of organismal energy homeostasis. It also investigates the molecular pathophysiology of metabolic diseases such as diabetes and obesity, as well as their treatment. Nature Metabolism follows the standards of other Nature-branded journals, with a dedicated team of professional editors, rigorous peer-review process, high standards of copy-editing and production, swift publication, and editorial independence. The journal has a high impact factor, has a certain influence in the international area, and is deeply concerned and cited by the majority of scholars.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


