Divergent synthesis of two polycyclic frameworks containing tricyclic bridgehead carbon centers by gold-catalyzed cycloisomerization of o-cyclopropylidenemethyl-o′-alkynylbiaryls
IF 4.7
1区 化学
Q1 CHEMISTRY, ORGANIC
引用次数: 0
Abstract
Compounds containing tricyclic bridgehead carbon centers are privileged structures in drug discovery. In this work, two different polycyclic scaffolds containing this substructure have been accessed by divergent gold-catalyzed cycloisomerizations of o-cyclopropylidenemethyl-o′-alkynylbiaryls. Selectivity towards one or the other scaffold is mainly controlled by temperature. The electronic nature of the arene group at the alkyne also plays a significant role, which is explained based on the proposed mechanism.
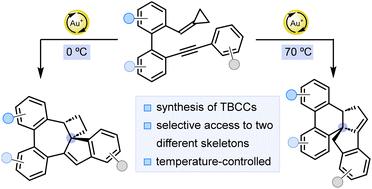
金催化邻环丙基甲基-o′-炔基二芳基环异构化合成含三环桥头堡碳中心的两个多环框架
含有三环桥头堡碳中心的化合物是药物发现中的优势结构。在这项工作中,含有该亚结构的两种不同的多环支架已经通过不同的金催化的o-环丙基甲基-o ' -炔基二芳基的环异构化得到。对一种或另一种支架的选择性主要由温度控制。炔上芳烃基团的电子性质也起着重要的作用,这是根据所提出的机理来解释的。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Organic Chemistry Frontiers
CHEMISTRY, ORGANIC-
CiteScore
7.90
自引率
11.10%
发文量
686
审稿时长
1 months
期刊介绍:
Organic Chemistry Frontiers is an esteemed journal that publishes high-quality research across the field of organic chemistry. It places a significant emphasis on studies that contribute substantially to the field by introducing new or significantly improved protocols and methodologies. The journal covers a wide array of topics which include, but are not limited to, organic synthesis, the development of synthetic methodologies, catalysis, natural products, functional organic materials, supramolecular and macromolecular chemistry, as well as physical and computational organic chemistry.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


