Aligning personalized biomarker trajectories onto a common time axis: a connectome-based ODE model for Tau–Amyloid beta dynamics
IF 11.8
1区 医学
Q1 COMPUTER SCIENCE, ARTIFICIAL INTELLIGENCE
引用次数: 0
Abstract
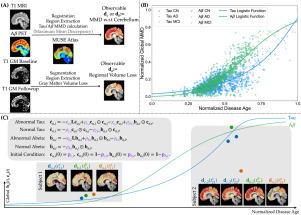
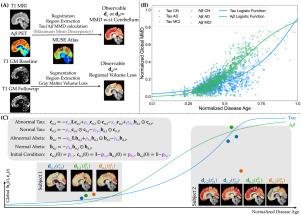
Abnormal tau and amyloid beta are two primary imaging biomarkers used to assist in the diagnosis of Alzheimer’s disease (AD). Recent efforts have focused on developing mechanism-based biophysical models to explain the spatiotemporal dynamics of these biomarkers. In this study, we adopt a connectome-based ODE model to capture the dynamics of tau and amyloid beta (), aiming to predict personalized future values of these biomarkers. The ODE model includes diffusion, reaction, and clearance terms, and accounts for tau– interactions. Additionally, it assumes a sparse initial condition (IC) of abnormalities, based on the assumption of localized initiation. Besides tau and , brain atrophy is used as a marker of neurodegeneration. We discuss the mathematical model of atrophy integrated into the tau– model. A common limitation in popular models is the use of chronological age as the time axis, which prevents the unification of subject trajectories onto a common time scale and hinders comprehensive cohort analysis. To address this issue, we use a normalized disease age that relates chronological age to biomarker values. In the ODE model, we use the disease age to track time and the biomarker dynamics. Furthermore, our analysis of region-of-interest-wise tau– temporal correlation reveals that different regions of interest (ROIs) play distinct roles across various disease stages.
将个性化生物标志物轨迹对准共同的时间轴:tau -淀粉样蛋白动力学的基于连接体的ODE模型
异常的tau蛋白和β淀粉样蛋白是用于协助诊断阿尔茨海默病(AD)的两种主要成像生物标志物。最近的努力集中在开发基于机制的生物物理模型来解释这些生物标志物的时空动态。在这项研究中,我们采用基于连接体的ODE模型来捕捉tau和β淀粉样蛋白(a β)的动态,旨在预测这些生物标志物的个性化未来价值。ODE模型包括扩散,反应和清除项,并说明tau - a - β相互作用。此外,基于局部起始的假设,假设了异常的稀疏初始条件(IC)。除了tau和a β,脑萎缩被用作神经退行性变的标志。我们讨论了整合tau - a - β模型的萎缩数学模型。流行模型的一个共同限制是使用实足年龄作为时间轴,这阻碍了受试者轨迹在共同时间尺度上的统一,并阻碍了全面的队列分析。为了解决这个问题,我们使用了一个标准化的疾病年龄,将实足年龄与生物标志物值联系起来。在ODE模型中,我们使用疾病年龄来跟踪时间和生物标志物动态。此外,我们对感兴趣区域的tau - a - β时间相关性的分析表明,不同的感兴趣区域(roi)在不同的疾病阶段起着不同的作用。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Medical image analysis
工程技术-工程:生物医学
CiteScore
22.10
自引率
6.40%
发文量
309
审稿时长
6.6 months
期刊介绍:
Medical Image Analysis serves as a platform for sharing new research findings in the realm of medical and biological image analysis, with a focus on applications of computer vision, virtual reality, and robotics to biomedical imaging challenges. The journal prioritizes the publication of high-quality, original papers contributing to the fundamental science of processing, analyzing, and utilizing medical and biological images. It welcomes approaches utilizing biomedical image datasets across all spatial scales, from molecular/cellular imaging to tissue/organ imaging.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


