Ni-induced modulation of Pt 5d–H 1s antibonding orbitals for enhanced hydrogen evolution and urea oxidation
IF 13.5
2区 化学
Q1 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
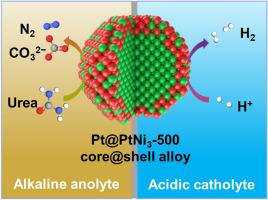
While H2 features high energy density, environmental friendliness, and renewability, its efficient production is limited by the sluggish kinetics of the oxygen evolution reaction (OER). Here, we report a Pt@PtNi3 core@shell alloy electrocatalyst that, through Ni incorporation, modulates the occupancy of Pt 5d antibonding orbitals and simultaneously enhances both hydrogen evolution reaction (HER) and urea oxidation reaction (UOR) activities. The optimized Pt@PtNi3-500 delivers an ultralow overpotential of 21 mV at 10 mA cm−2 for HER under acidic conditions and a low onset potential of 1.27 V for UOR under alkaline conditions, surpassing monometallic Pt and Ni counterparts. When employed in an asymmetric acid-alkaline electrolyzer (HER/UOR), Pt@PtNi3-500 achieves a 68.3 % reduction in electrical energy consumption for H2 production compared to traditional alkaline water splitting (HER/OER). Mechanistic investigations reveal that appropriate Ni incorporation in Pt@PtNi3 increases the occupancy of Pt 5d–H 1s antibonding orbitals, which not only reinforces H+ adsorption but also weakens the overly strong H∗ binding. Simultaneously, it reduces the energy barrier for ∗NH2 dehydrogenation, thereby synergistically accelerating both H2 generation and urea decomposition. This work provides new insights into the design of alloy electrocatalysts for high-efficiency H2 production.
镍诱导的Pt 5d-H - 1s反键轨道的调制促进了析氢和尿素氧化
虽然H2具有高能量密度、环境友好和可再生的特点,但其高效生产受到析氧反应(OER)动力学缓慢的限制。在这里,我们报道了一种Pt@PtNi3 core@shell合金电催化剂,通过Ni的加入,调节Pt 5d反键轨道的占用,同时提高析氢反应(HER)和尿素氧化反应(UOR)的活性。优化后的Pt@PtNi3-500在酸性条件下为HER提供了21 mV、10 mA cm−2的超低过电位,在碱性条件下为UOR提供了1.27 V的低起始电位,超过了单金属Pt和Ni。当在不对称酸碱性电解槽(HER/UOR)中使用时,Pt@PtNi3-500与传统的碱性水分解(HER/OER)相比,H2生产的电能消耗降低了68.3%。机制研究表明,Pt@PtNi3中适当的Ni掺入增加了Pt 5d-H 1s反键轨道的占用,这不仅加强了H+的吸附,而且减弱了过强的H *结合。同时,它降低了NH2脱氢的能垒,从而协同加速H2生成和尿素分解。本研究为高效制氢合金电催化剂的设计提供了新的思路。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


