Yellow fever virus infection triggers proinflammatory and prothrombotic responses in endothelial cells through NF-κB and MAPK signaling pathways
IF 3.4
3区 医学
Q1 HEMATOLOGY
引用次数: 0
Abstract
Yellow fever (YF), caused by the Yellow Fever Virus (YFV), is a disease endemic in South America and Africa with clinical manifestations ranging from fever to fatal organ failure and hemorrhagic complications. The role of the endothelium in the pathogenesis of YFV is not completely understood. To investigate the effects of YFV infection on human endothelial cells (EC), human umbilical vein endothelial cells (HUVEC) and human microvascular endothelial cells (HMEC-1) were infected with the YFV 17DD strain. Viral infection, cell death, cell adhesion, adhesion molecules, cytokines, von Willebrand factor (VWF), nitric oxide (NO), tissue factor (TF), interferon-I, clotting time and signaling pathways were analyzed by plaque assays, immunofluorescence, cytometry, ELISA, RT-qPCR, DAF-FM DA probe, Griess reaction and Western blot.
The results showed that HUVEC and HMEC-1 were susceptible to YFV infection according to virus titer and presence of intracellular dsRNA, which induced an increase in apoptosis at 3- and 7-days post-infection (pi), respectively. At earlier time points (4–48 h pi), infected EC exhibited upregulation of E-selectin and ICAM-1, secretion of IL-1β, IL-6 and VWF, decreased eNOS mRNA, NO production, clotting time, and increased TF and interferon-I mRNA levels, as well as increased adhesion of platelets and leukocytes to EC. Mechanistically, YFV infection led to activation of the NF-κB and MAPK signaling pathways.
We conclude that YFV infection triggers a proinflammatory and prothrombotic response in EC that likely contributes to the vascular dysfunction and hemorrhagic manifestations observed in YF. These findings point to potential targets for therapeutic intervention.
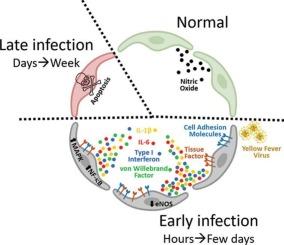
黄热病病毒感染通过NF-κB和MAPK信号通路触发内皮细胞的促炎和促血栓反应
黄热病由黄热病病毒(YFV)引起,是南美洲和非洲的一种地方病,临床表现从发热到致命性器官衰竭和出血性并发症。内皮在YFV发病机制中的作用尚不完全清楚。为探讨YFV感染对人内皮细胞(EC)的影响,采用YFV 17DD株感染人脐静脉内皮细胞(HUVEC)和人微血管内皮细胞(HMEC-1)。采用斑块检测、免疫荧光、细胞计数、ELISA、RT-qPCR、DAF-FM DA探针、Griess反应、Western blot等方法分析病毒感染、细胞死亡、细胞粘附、粘附分子、细胞因子、von Willebrand factor (VWF)、一氧化氮(NO)、组织因子(TF)、干扰素- i、凝血时间和信号通路。结果显示,根据病毒滴度和细胞内dsRNA的存在,HUVEC和HMEC-1对YFV感染敏感,分别在感染后3天和7天(pi)诱导凋亡增加。在较早的时间点(4 - 48h),感染EC表现出e -选择素和ICAM-1的上调,IL-1β、IL-6和VWF的分泌,eNOS mRNA、NO产生、凝血时间减少,TF和干扰素-1 mRNA水平升高,血小板和白细胞对EC的粘附增加。在机制上,YFV感染导致NF-κB和MAPK信号通路的激活。我们得出结论,YFV感染在EC中引发了促炎和促血栓反应,这可能导致了YF中观察到的血管功能障碍和出血表现。这些发现指出了治疗干预的潜在目标。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Thrombosis research
医学-外周血管病
CiteScore
14.60
自引率
4.00%
发文量
364
审稿时长
31 days
期刊介绍:
Thrombosis Research is an international journal dedicated to the swift dissemination of new information on thrombosis, hemostasis, and vascular biology, aimed at advancing both science and clinical care. The journal publishes peer-reviewed original research, reviews, editorials, opinions, and critiques, covering both basic and clinical studies. Priority is given to research that promises novel approaches in the diagnosis, therapy, prognosis, and prevention of thrombotic and hemorrhagic diseases.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


