Oxidative stress-induced ZEB1 acetylation drives a hybrid epithelial-mesenchymal phenotype and promotes lung metastasis in triple-negative breast cancer
IF 11.9
1区 生物学
Q1 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
Abstract
While epithelial-mesenchymal plasticity (EMP) drives cancer metastasis, its regulation by redox dynamics remains poorly understood. Herein, we identified an oxidative stress-responsive CBP/SIRT1 axis that coordinated ZEB1 acetylation at K1108 to promote lung metastasis in triple-negative breast cancer (TNBC). Mechanistically, the biochemical and functional analyses revealed that the dual-acetyltransferase CBP, through stabilization and autoacetylation by oxidative stress, formed a dynamic partnership with SIRT1 to execute precision lysine modification. This post-translational rheostat triggered the functional metamorphosis of ZEB1. During this process, ZEB1 dissociation from the transcriptional corepressor CtBP, while recruiting CBP, converts ZEB1 into a transcriptional activator of epithelial genes. The resulting hybrid epithelial‒mesenchymal phenotype orchestrated dual metastatic competence-maintaining stromal interaction capacity through partial epithelial‒mesenchymal transition (EMT) while establishing NADPH-driven redox supremacy to circumvent ferroptosis. Importantly, this acetyl switch of ZEB1 revealed a metastasis-specific therapeutic vulnerability in TNBC. Our work thus highlighted ZEB1 acetylation as a redox-interpreted mechanism coupling phenotypic plasticity with stress resistance, proposing targeted disruption of this protein post-translational modification (PTM) circuit as a precision strategy against metastatic progression.
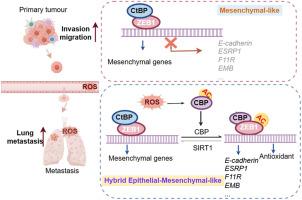
氧化应激诱导的ZEB1乙酰化驱动三阴性乳腺癌的上皮-间质杂交表型并促进肺转移
虽然上皮-间充质可塑性(EMP)驱动癌症转移,但其通过氧化还原动力学的调控仍然知之甚少。在此,我们发现了一个氧化应激响应的CBP/SIRT1轴,它在K1108位点协调ZEB1乙酰化,促进三阴性乳腺癌(TNBC)的肺转移。机制上,生化和功能分析表明,双乙酰转移酶CBP通过氧化应激的稳定和自乙酰化,与SIRT1形成动态伙伴关系,进行精确的赖氨酸修饰。这种翻译后变阻器触发了ZEB1的功能变态。在此过程中,ZEB1与转录辅抑制因子CtBP分离,同时募集CBP,将ZEB1转化为上皮基因的转录激活因子。由此产生的杂交上皮-间充质表型通过部分上皮-间充质转化(EMT)协调了双重转移能力-维持基质相互作用能力,同时建立了nadph驱动的氧化还原优势以避免铁凋亡。重要的是,ZEB1的乙酰化开关揭示了TNBC中转移特异性的治疗脆弱性。因此,我们的工作强调了ZEB1乙酰化是一种氧化还原解释的机制,将表型可塑性与抗逆性耦合在一起,并提出了靶向破坏这种蛋白质翻译后修饰(PTM)电路作为一种精确的策略来防止转移进展。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Redox Biology
BIOCHEMISTRY & MOLECULAR BIOLOGY-
CiteScore
19.90
自引率
3.50%
发文量
318
审稿时长
25 days
期刊介绍:
Redox Biology is the official journal of the Society for Redox Biology and Medicine and the Society for Free Radical Research-Europe. It is also affiliated with the International Society for Free Radical Research (SFRRI). This journal serves as a platform for publishing pioneering research, innovative methods, and comprehensive review articles in the field of redox biology, encompassing both health and disease.
Redox Biology welcomes various forms of contributions, including research articles (short or full communications), methods, mini-reviews, and commentaries. Through its diverse range of published content, Redox Biology aims to foster advancements and insights in the understanding of redox biology and its implications.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


