An improved and economical LLE-LC/MS method for quantifying phosphatidylethanol: Identification and separation of isotopic cross-talk in clinical alcohol testing
IF 2.1
3区 医学
Q2 MEDICAL LABORATORY TECHNOLOGY
引用次数: 0
Abstract
Objective
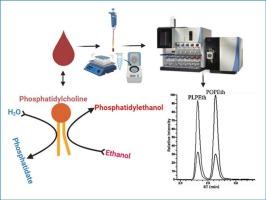
Phosphatidylethanol (PEth) is a group of phospholipids formed in the presence of ethanol on the red blood cell membrane. Due to their stability in blood for 3–4 weeks, they have become reliable direct biomarkers for long-term monitoring of alcohol use. This study aimed to develop and validate a robust, high-throughput liquid chromatography and tandem mass spectrometry (LC-MS/MS) method for the routine clinical quantification of the two most common PEth homologues, PEth 16:0/18:1 (POPEth) and PEth 16:0/18:2 (PLPEth), while addressing common analytical challenges.
Methods
An established quantification method employing liquid–liquid extraction was used with optimized LC-MS/MS parameters. The method was validated for correlation studies, precision, analytical measurement range, analytical sensitivity, analytical specificity, carryover, dilution linearity, stability, matrix effect and extraction recovery, with specific attention to eliminating isotopic cross-talk and chromatographic interferences. A method comparison was performed using specimens analyzed by an external reference laboratory.
Results
The method demonstrated excellent linearity for both POPEth and PLPEth across the analytical measurement range (10–2000 ng/mL), with correlation coefficients (r2) of 0.99. Intra- and inter-assay precision values were within ± 10 % coefficient of variation. Recovery yields ranged from 78-85 %. The optimized method resolved isotopic cross-talk and exhibited no carryover. Comparison with an external laboratory showed strong correlation for both homologues (slopes of 0.979 and 1.049; r2 = 0.99).
Conclusion
We developed and validated a sensitive and specific LC-MS/MS method for the quantification of POPEth and PLPEth. The assay provides improved recovery, eliminates isotopic cross-talk, shows no carryover, and is suitable for high-throughput clinical laboratories. This method enables reliable and cost-effective monitoring of alcohol use in routine clinical practice.
一种改进且经济的液相色谱-质谱法定量磷脂酰乙醇:临床酒精检测中同位素串扰的鉴定与分离
目的:磷脂酰乙醇(PEth)是在乙醇存在下在红细胞膜上形成的一组磷脂。由于它们在血液中的稳定性为3-4周,它们已成为长期监测酒精使用情况的可靠直接生物标志物。本研究旨在开发和验证一种强大的高通量液相色谱-串联质谱(LC-MS/MS)方法,用于常规临床定量两种最常见的PEth同源物,PEth 16:0/18:1 (POPEth)和PEth 16:0/18:2 (PLPEth),同时解决常见的分析挑战。方法采用优化的LC-MS/MS参数,建立液液萃取定量方法。验证了该方法的相关性研究、精密度、分析测量范围、分析灵敏度、分析特异性、携带、稀释线性、稳定性、基质效应和提取回收率,特别注意消除同位素串扰和色谱干扰。采用外部参比实验室分析的标本进行方法比较。结果POPEth和PLPEth在10 ~ 2000 ng/mL的分析范围内呈良好的线性关系,相关系数(r2)为0.99。测定内和测定间的精密度值在±10%变异系数内。回收率为78 ~ 85%。优化后的方法解决了同位素串扰,无遗留现象。与外部实验室比较,两种同源物具有较强的相关性(斜率分别为0.979和1.049,r2 = 0.99)。结论建立了一种灵敏、特异的hplc -MS/MS定量方法。该检测方法提高了回收率,消除了同位素串扰,无残留,适用于高通量临床实验室。这种方法能够在常规临床实践中可靠和具有成本效益地监测酒精使用情况。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Clinical biochemistry
医学-医学实验技术
CiteScore
5.10
自引率
0.00%
发文量
151
审稿时长
25 days
期刊介绍:
Clinical Biochemistry publishes articles relating to clinical chemistry, molecular biology and genetics, therapeutic drug monitoring and toxicology, laboratory immunology and laboratory medicine in general, with the focus on analytical and clinical investigation of laboratory tests in humans used for diagnosis, prognosis, treatment and therapy, and monitoring of disease.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


