Long-term intermittent theta burst stimulation alleviates Parkinson's disease-related cognitive impairment by modulating GluN2B in the dorsal hippocampus
IF 4.2
2区 医学
Q1 NEUROSCIENCES
引用次数: 0
Abstract
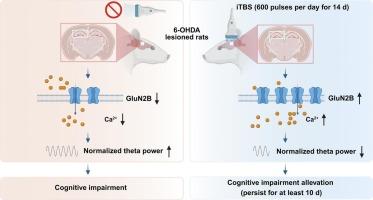
Cognitive impairment is one of the typical non-motor symptoms of Parkinson's disease (PD) that severely affects the quality of life of patients. However, limited treatments are currently available, suggesting the urgent need for new therapeutic approaches. Intermittent theta burst stimulation (iTBS), an updated pattern of high-frequency repetitive transcranial magnetic stimulation, can potentially improve cognitive function. However, its efficacy on PD-related cognitive impairment and the mechanism underlying it remain unclear. In this study, we found that unilateral 6-hydroxydopamine (6-OHDA) lesions of the substantia nigra pars compacta (SNc) impaired hippocampus-dependent memory, decreased the expression of GluN2B at both the total and membrane protein levels, reduced the concentration of intracellular Ca2+, and resulted in hyperactive theta power in the dorsal hippocampus (dHip) in rats. Fourteen days of iTBS treatment improved the impaired hippocampus-dependent memory in the lesioned rats, which could last for at least 10 days. In addition, iTBS treatment up-regulated the expression of GluN2B at total and membrane protein levels, elevated intracellular Ca2+ concentration, and normalized the aberrantly high theta power in the dHip. Furthermore, iTBS treatment failed to improve hippocampus-dependent memory and normalize the aberrant theta power after knocking down the hippocampal GluN2B. Collectively, these findings suggest that 14-day iTBS treatment alleviates hippocampus-dependent memory impairment in PD, which is achieved by up-regulating the expression of the GluN2B, followed by increasing the level of intracellular Ca2+ concentration and normalizing hyperactive theta rhythm in the dHip.
长期间歇性θ波爆发刺激通过调节海马背侧GluN2B减轻帕金森病相关认知障碍
认知障碍是帕金森病(PD)典型的非运动症状之一,严重影响患者的生活质量。然而,目前可用的治疗方法有限,这表明迫切需要新的治疗方法。间歇性θ波爆发刺激(iTBS)是高频重复经颅磁刺激的一种更新模式,可以潜在地改善认知功能。然而,其对pd相关认知障碍的疗效及其机制尚不清楚。本研究发现,大鼠黑质致密部(SNc)单侧6-羟多巴胺(6-OHDA)损伤会损害海马依赖记忆,降低GluN2B在总蛋白和膜蛋白水平上的表达,降低细胞内Ca2+浓度,导致海马背侧(dHip) θ波功率过度活跃。14天的iTBS治疗改善了受损大鼠的海马依赖记忆,这种情况可能持续至少10天。此外,iTBS处理上调了GluN2B在总蛋白和膜蛋白水平上的表达,提高了细胞内Ca2+浓度,并使dHip中异常高的θ波功率正常化。此外,iTBS治疗未能改善海马依赖性记忆,并在敲除海马GluN2B后使异常的θ波能量正常化。综上所述,这些发现表明,14天的iTBS治疗可以减轻PD患者海马依赖性记忆障碍,这是通过上调GluN2B的表达,随后增加细胞内Ca2+浓度水平和使dHip中过度活跃的theta节律正常化来实现的。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Experimental Neurology
医学-神经科学
CiteScore
10.10
自引率
3.80%
发文量
258
审稿时长
42 days
期刊介绍:
Experimental Neurology, a Journal of Neuroscience Research, publishes original research in neuroscience with a particular emphasis on novel findings in neural development, regeneration, plasticity and transplantation. The journal has focused on research concerning basic mechanisms underlying neurological disorders.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


