Respiratory-related safety profiles of ciprofol (cipepofol) for anesthesia/sedation in Chinese elderly patients undergoing gastroscopy: a multicenter, parallel controlled clinical trial (REST trial)
IF 5.1
2区 医学
Q1 ANESTHESIOLOGY
引用次数: 0
Abstract
Background
A post-marketing, parallel-controlled clinical trial (REST trial) was conducted to evaluate the safety and efficacy of cipepofol versus propofol for the induction of anesthesia/sedation in Chinese elderly patients undergoing gastroscopy.
Methods
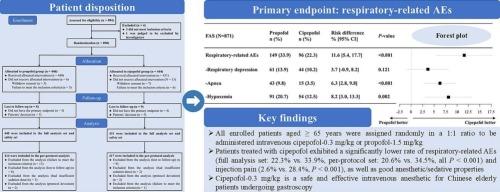
All enrolled patients aged ≥65 years were assigned randomly in a 1:1 ratio to be administered intravenous cipepofol-0.3 mg/kg or propofol-1.5 mg/kg. The primary endpoint was incidence of respiratory-related adverse events (AEs) including respiratory depression (respiratory rate < 8 breaths/min lasting for >30 s), apnea (loss of thoracic movement for >15 s) and hypoxemia (SpO2 < 93 % lasting for >15 s). Secondary endpoints included: success rates of the gastroscopy procedure and gastroscope insertion; gastroscopy-related duration (successful anesthesia/sedation induction duration; time to full alertness; time to leaving the post-anesthesia care unit; gastroscope insertion duration); satisfaction rate for the anesthesia/sedation process and anesthetics.
Results
Among 890 randomized patients, 871 were finally included in the full analysis set (FAS), with 431 receiving cipepofol and 440 receiving propofol. Patients treated with cipepofol had a significantly lower incidence of respiratory-related AEs compared to propofol treatment (FAS: 22.3 % vs. 33.9 %, per-protocol set: 20.6 % vs. 34.5 %, all P < 0.001), regardless of sex. Multivariable analysis revealed that the risk of patients experiencing respiratory-related AEs was 1.82 times higher in the propofol group compared to cipepofol group (P < 0.001). The success rates of the gastroscopy procedure and gastroscope insertion were both 100 % in the two groups. Gastroscopy procedure-related durations were shorter in propofol group compared to cipepofol group (all P < 0.05). Patients treated with cipepofol exhibited a significantly lower rate of treatment-emergent AEs (TEAEs) (55.0 % vs. 67.7 %, P < 0.001), TEAEs of special interest (53.4 % vs. 65.2 %, P < 0.001) and injection pain (2.6 % vs. 28.4 %, P < 0.001).
Conclusions
Cipepofol-0.3 mg/kg is a safe and effective intravenous anesthetic for Chinese elderly patients undergoing gastroscopy, especially complimented by lower incidences of respiratory-related AEs and injection pain.
Clinical trials registration: Chinese Clinical Trial Registry, ChiCTR2100052299, registered on October 24, 2021.
环丙酚(cipepofol)用于中国老年胃镜患者麻醉/镇静的呼吸相关安全性:一项多中心平行对照临床试验(REST试验)
本研究开展了一项上市后平行对照临床试验(REST试验),以评价西泊酚与异丙酚在中国老年胃镜患者诱导麻醉/镇静中的安全性和有效性。方法所有年龄≥65岁的患者按1:1的比例随机分配给静脉注射西哌泊酚0.3 mg/kg或异丙酚1.5 mg/kg。主要终点是呼吸相关不良事件(ae)的发生率,包括呼吸抑制(呼吸频率8次/分钟,持续30秒)、呼吸暂停(胸部运动丧失,持续15秒)和低氧血症(SpO2, 93%,持续15秒)。次要终点包括:胃镜检查和胃镜插入的成功率;胃镜相关持续时间(成功麻醉/镇静诱导持续时间;达到完全清醒的时间;离开麻醉后护理病房的时间;插入胃镜的时间);麻醉/镇静过程及麻醉药满意率。结果890例随机患者中,871例最终纳入全分析集(FAS),其中431例接受西泊酚治疗,440例接受异丙酚治疗。与异丙酚治疗相比,接受西泊酚治疗的患者呼吸相关不良事件的发生率显著降低(FAS: 22.3% vs. 33.9%,每方案集:20.6% vs. 34.5%,均P <; 0.001),与性别无关。多变量分析显示,异丙酚组患者发生呼吸相关不良事件的风险是西哌泊酚组的1.82倍(P < 0.001)。两组胃镜手术及胃镜插入成功率均为100%。异丙酚组胃镜检查相关时间较异丙酚组短(P < 0.05)。使用西泊酚治疗的患者出现急性发作性不良反应(teae)的比率(55.0%比67.7%,P < 0.001)、特别关注的急性发作性不良反应(53.4%比65.2%,P < 0.001)和注射痛(2.6%比28.4%,P < 0.001)的比率显著降低。结论西泊酚0.3 mg/kg是一种安全有效的静脉麻醉药,适用于中国老年胃镜检查患者,特别是呼吸相关不良反应和注射痛的发生率较低。临床试验注册:中国临床试验注册中心,ChiCTR2100052299,于2021年10月24日注册。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
7.40
自引率
4.50%
发文量
346
审稿时长
23 days
期刊介绍:
The Journal of Clinical Anesthesia (JCA) addresses all aspects of anesthesia practice, including anesthetic administration, pharmacokinetics, preoperative and postoperative considerations, coexisting disease and other complicating factors, cost issues, and similar concerns anesthesiologists contend with daily. Exceptionally high standards of presentation and accuracy are maintained.
The core of the journal is original contributions on subjects relevant to clinical practice, and rigorously peer-reviewed. Highly respected international experts have joined together to form the Editorial Board, sharing their years of experience and clinical expertise. Specialized section editors cover the various subspecialties within the field. To keep your practical clinical skills current, the journal bridges the gap between the laboratory and the clinical practice of anesthesiology and critical care to clarify how new insights can improve daily practice.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


