The simultaneous activation of μ- and δ-opioid receptors by (−)-2S-LP2 rescues allodynia with the contribution of TGF-β1 signaling in a rat chronic constriction injury model
Q2 Agricultural and Biological Sciences
Current Research in Pharmacology and Drug Discovery
Pub Date : 2025-01-01
DOI:10.1016/j.crphar.2025.100229
引用次数: 0
Abstract
In neuropathic pain (NP), a dysregulation of glial functions in the central and peripheral nervous systems has been described, and the balance between pro-inflammatory and anti-inflammatory mediators is lost in the transition from acute to chronic pain. This raises the possibility to resolve pain via the induction of anti-inflammatory cytokines that have a protective role against neuroinflammatory events. Transforming growth factor-β1 (TGF-β1), an anti‐inflammatory cytokine is able to counteract the development of chronic NP. Given the correlation between opioid agonists and TGF-β1 pathway, here we describe the pharmacological profile of the dual-target μ-opioid receptor (MOR)/δ-opioid receptor (DOR) agonist (−)-2S-LP2. (−)-2S-LP2, given intraperitoneally at a dose of 0.7 mg/kg, significantly mitigated mechanical allodynia induced by chronic constriction injury in rats. This antiallodynic effect was sensitive to subcutaneous (s.c.) injection of either the MOR-selective antagonist naloxonazine (NLX, 10 mg/kg) or the DOR-selective antagonist naltrindole (NTD, 3 mg/kg), alone or when combined, demonstrating that (−)-2S-LP2 interacted simultaneously with both MOR and DOR. At mRNA or protein level, a positive effect on TGF-β1 and its receptor TGFβ-R2 expression were found and (−)-2S-LP2 also modulated the expression of spinal TGF-β1 pathway via co‐targeting MOR/DOR. Thus, the dual‐target profile of the MOR/DOR agonist (−)-2S-LP2 exerts its analgesic efficacy by rescue of TGF-β1 and could represent a novel pharmacological tool able to increase anti-inflammatory cytokines in pain conditions such as NP associated with an imbalance between inflammatory and anti-inflammatory cytokines.
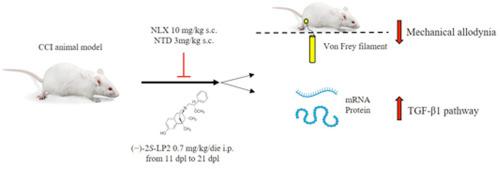
(−)- 2s - lp2同时激活μ-和δ-阿片受体,通过TGF-β1信号的作用,在大鼠慢性收缩损伤模型中缓解异位性疼痛
在神经性疼痛(NP)中,中枢和周围神经系统的神经胶质功能失调已经被描述,在从急性到慢性疼痛的过渡中,促炎和抗炎介质之间的平衡丢失。这增加了通过诱导抗炎细胞因子来解决疼痛的可能性,抗炎细胞因子对神经炎症事件具有保护作用。转化生长因子-β1 (TGF-β1)是一种抗炎细胞因子,能够抑制慢性NP的发展。鉴于阿片受体激动剂与TGF-β1通路之间的相关性,本文描述了双靶点μ-阿片受体(MOR)/δ-阿片受体(DOR)激动剂(−)-2S-LP2的药理学特征。(−)-2S-LP2以0.7 mg/kg的剂量腹腔注射,可显著减轻大鼠慢性收缩损伤引起的机械性异常痛。这种抗异动作用对皮下注射MOR选择性拮抗剂纳洛唑嗪(NLX, 10 mg/kg)或DOR选择性拮抗剂纳曲多(NTD, 3 mg/kg)均敏感,表明(−)-2S-LP2同时与MOR和DOR相互作用。在mRNA或蛋白水平上,我们发现TGF-β1及其受体TGF-β - r2的表达有正向影响,(−)-2S-LP2也通过共靶向MOR/DOR调节脊柱TGF-β1通路的表达。因此,MOR/DOR激动剂(−)-2S-LP2的双靶点特征通过挽救TGF-β1发挥其镇痛作用,可能代表一种新的药理学工具,能够增加疼痛条件下的抗炎细胞因子,如与炎症和抗炎细胞因子之间不平衡相关的NP。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Current Research in Pharmacology and Drug Discovery
Agricultural and Biological Sciences-Animal Science and Zoology
CiteScore
6.40
自引率
0.00%
发文量
65
审稿时长
40 days
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


