Sustainable synthesis of 2,4-diarylquinoline derivatives using recyclable BiFeO3 nanocatalyst in ionic liquid
IF 2.1
3区 化学
Q3 CHEMISTRY, INORGANIC & NUCLEAR
引用次数: 0
Abstract
In continuation of our program, the purpose of the present investigation was to develop a highly efficient and eco-friendly catalytic system comprising bismuth ferrite nanoparticles (BiFeO3 NPs) in an ionic liquid in a single-pot for the production of 2,4-diarylquinoline derivatives through a three-part reaction of aromatic aldehydes, alkynes, and aniline derivatives. FTIR, SEM, TEM, TGA, BET, VSM, XRD, EDX, and elemental mapping were used in order to create and elucidate the structural and magnetic properties of the BiFeO3 nanoparticles. The catalyst demonstrated excellent reusability, in line with green chemistry principles, maintaining its activity for at least eight consecutive cycles. High catalytic activity, compliance with green chemistry principles, and excellent recyclability of BiFeO3 nanoparticles, which can be reused up to 8 times without loss of catalytic activity, are some of the advantages of this approach. The catalyst's magnetic property can facilitate separation and recycling, while the use of ionic liquids as reaction solvents is environmentally benign. The system's capacity to operate in moderate conditions and support a wide variety of substrates enables the synthesis of 2,4-diarylquinoline derivatives, which are both versatile and feasible.
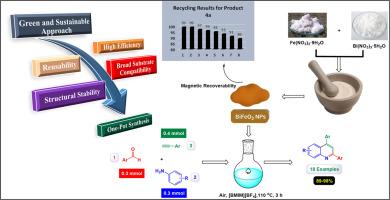
离子液体中可回收BiFeO3纳米催化剂可持续合成2,4-二芳基喹啉衍生物
为了继续我们的计划,本研究的目的是在单锅中开发一种高效环保的催化系统,该系统由离子液体中的铋铁氧体纳米粒子(BiFeO3 NPs)组成,用于通过芳香醛、炔和苯胺衍生物的三组分反应生产2,4-二芳基喹啉衍生物。利用FTIR、SEM、TEM、TGA、BET、VSM、XRD、EDX和元素映射等方法对BiFeO3纳米粒子的结构和磁性进行了表征。该催化剂表现出优异的可重复使用性,符合绿色化学原则,保持其活性至少连续8个循环。高催化活性,符合绿色化学原理,并且BiFeO3纳米颗粒具有优异的可回收性,可以重复使用多达8次而不损失催化活性,这是该方法的一些优点。催化剂的磁性可以促进分离和回收,而使用离子液体作为反应溶剂是环保的。该系统在中等条件下运行和支持多种底物的能力使2,4-二芳基喹啉衍生物的合成成为可能,既通用又可行。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Organometallic Chemistry
化学-无机化学与核化学
CiteScore
4.40
自引率
8.70%
发文量
221
审稿时长
36 days
期刊介绍:
The Journal of Organometallic Chemistry targets original papers dealing with theoretical aspects, structural chemistry, synthesis, physical and chemical properties (including reaction mechanisms), and practical applications of organometallic compounds.
Organometallic compounds are defined as compounds that contain metal - carbon bonds. The term metal includes all alkali and alkaline earth metals, all transition metals and the lanthanides and actinides in the Periodic Table. Metalloids including the elements in Group 13 and the heavier members of the Groups 14 - 16 are also included. The term chemistry includes syntheses, characterizations and reaction chemistry of all such compounds. Research reports based on use of organometallic complexes in bioorganometallic chemistry, medicine, material sciences, homogeneous catalysis and energy conversion are also welcome.
The scope of the journal has been enlarged to encompass important research on organometallic complexes in bioorganometallic chemistry and material sciences, and of heavier main group elements in organometallic chemistry. The journal also publishes review articles, short communications and notes.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


