p53 promote oxidative stress, neuroinflammation and behavioral disorders via DDIT4-NF-κB signaling pathway
IF 11.9
1区 生物学
Q1 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
Abstract
Depression is a mood disorder characterized by persistent emotional and behavioral dysregulation. Oxidative stress-induced neuronal damage is increasingly recognized as a critical risk factor contributing to the pathogenesis of depression. However, the potential molecular mechanisms and therapeutic targets underlying brain homeostasis disruption induced by neuroinflammatory responses remain unclear. The polyphenolic compound curcumin has been shown to exert neuroprotective effects and partially alleviate depression-related behavioral symptoms through its anti-oxidative properties. However, the molecular mechanisms and therapeutic targets underlying curcumin's ability to ameliorate oxidative stress-induced behavioral abnormalities in specific brain regions remain insufficiently defined. In this study, we demonstrate that chronic administration of corticosterone (CORT) induces pronounced depression- and anxiety-like behaviors in mice, accompanied by marked oxidative stress, neuroinflammation, and disrupted synaptic plasticity within the medial prefrontal cortex (mPFC). Curcumin treatment significantly ameliorated these behavioral and neuropathological abnormalities by enhancing antioxidant capacity, suppressing inflammatory cytokine production and restoring dendritic architecture. Transcriptomic profiling and network pharmacology identified the p53-DDIT4-NF-κB signaling as a key signaling hub underlying these effects. Pharmacological inhibition of p53 with pifithrin-α (PFT-α) mimicked the antidepressant-like effects of curcumin, whereas activation with NSC697923 abolished them. These findings support curcumin may serve as a promising strategy for anti-oxidative stress and anti-neuroinflammation in depression via targeting p53-DDIT4-NF-κB signaling.
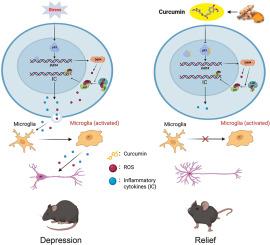
p53通过DDIT4-NF-κB信号通路促进氧化应激、神经炎症和行为障碍
抑郁症是一种以持续的情绪和行为失调为特征的情绪障碍。氧化应激诱导的神经元损伤越来越被认为是抑郁症发病机制的一个重要危险因素。然而,神经炎症反应引起的脑内平衡破坏的潜在分子机制和治疗靶点尚不清楚。多酚类化合物姜黄素已被证明具有神经保护作用,并通过其抗氧化特性部分缓解抑郁相关的行为症状。然而,姜黄素改善氧化应激诱导的特定脑区行为异常的分子机制和治疗靶点仍不明确。在这项研究中,我们证明了长期给药皮质酮(CORT)在小鼠中诱导明显的抑郁和焦虑样行为,伴随着明显的氧化应激、神经炎症和内侧前额叶皮层(mPFC)突触可塑性的破坏。姜黄素治疗通过增强抗氧化能力、抑制炎症细胞因子的产生和恢复树突结构,显著改善了这些行为和神经病理异常。转录组学分析和网络药理学鉴定p53-DDIT4-NF-κB信号是这些作用的关键信号中枢。聚氟氰菊酯-α (PFT-α)对p53的药理抑制作用类似姜黄素的抗抑郁作用,而NSC697923的激活则可以消除这种作用。这些发现支持姜黄素可能通过靶向p53-DDIT4-NF-κB信号通路,作为抗氧化应激和抗抑郁症神经炎症的有希望的策略。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Redox Biology
BIOCHEMISTRY & MOLECULAR BIOLOGY-
CiteScore
19.90
自引率
3.50%
发文量
318
审稿时长
25 days
期刊介绍:
Redox Biology is the official journal of the Society for Redox Biology and Medicine and the Society for Free Radical Research-Europe. It is also affiliated with the International Society for Free Radical Research (SFRRI). This journal serves as a platform for publishing pioneering research, innovative methods, and comprehensive review articles in the field of redox biology, encompassing both health and disease.
Redox Biology welcomes various forms of contributions, including research articles (short or full communications), methods, mini-reviews, and commentaries. Through its diverse range of published content, Redox Biology aims to foster advancements and insights in the understanding of redox biology and its implications.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


