Quantitative proteomic data of flotillin-2 interactome within detergent resistant membranes of HeLa cells
IF 1.4
Q3 MULTIDISCIPLINARY SCIENCES
引用次数: 0
Abstract
Flotillin-binding protein networks serve as scaffolds, organizing lipid rafts and facilitating the recruitment of other raft-associated proteins such as receptors and downstream signaling molecules to regulate various intracellular pathways, including those involved in cell proliferation, migration, and endocytosis.
Flotillins belong to the SPFH (stomatin/prohibitin/flotillin/HflK/C) domain-containing protein family, also known as the prohibitin homology (PHB) domain, which enables membrane association via acylation and hydrophobic hairpin motifs that anchor them to the inner leaflet of the plasma membrane. The functional diversity of flotillin proteins within membrane microdomains primarily stems from their interactions with other proteins.
Data presented in this article characterize the proximal interactome of flotillin-2 within detergent-resistant membranes (DRMs) using BioID, a proximity-dependent biotinylation technique. Flotillin-2 was fused with the biotin ligase BirA* at either the N- or C-terminus and expressed in HeLa cells. DRMs were isolated through sucrose density gradient ultracentrifugation, and biotinylated proteins were purified using biotin–avidin affinity followed by label-free quantitative (LFQ) mass spectrometry.
This approach identified a set of proteins significantly enriched in DRM fractions from cells expressing flotillin-2–BirA* fusions compared to control cells expressing BirA*–mEGFP. The two analyses allowed for relative quantification of ∼433 and ∼926 unique proteins across the N-terminal and C-terminal BirA* fusions, respectively. 28 (N-terminal) and 88 (C-terminal) proteins were observed as significantly enriched in flotillin-2 samples (28 and 43 with fold change ≥ 2). These enriched proteins are candidate interactors of flotillin-2 within membrane raft domains. Notably, the N-terminal fusion-associated proteins were significantly linked to specific biological processes such as dendritic transport and regulation of signal transduction, whereas the C-terminal fusion group showed enrichment in membrane biogenesis-related proteins. Among here presented DRM partners of flotillin-2, both previously known and earlier unreported interactors of this protein were found. Overall, our BioID-based analysis provides valuable insight into the flotillin-2 interactome in DRM fractions and lays the groundwork for future studies exploring the regulation of membrane lateral heterogeneity and the role of flotillin-mediated domains in signaling pathways, particularly those dysregulated in diseases such as cancer.
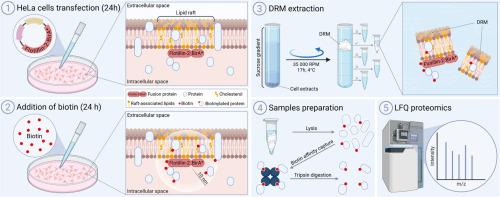
HeLa细胞耐洗涤膜中flotilin -2相互作用组的定量蛋白质组学数据
漂浮蛋白结合蛋白网络作为支架,组织脂筏并促进其他筏相关蛋白如受体和下游信号分子的募集,以调节各种细胞内通路,包括细胞增殖、迁移和内吞作用。Flotillins属于含有SPFH (stomatin/prohibitin/flotillin/HflK/C)结构域的蛋白家族,也被称为禁止蛋白同源性(PHB)结构域,它通过酰化和疏水发夹基元使膜结合,将它们固定在质膜的内小叶上。膜微域内漂浮蛋白的功能多样性主要源于它们与其他蛋白的相互作用。本文中提供的数据利用BioID(一种邻近依赖的生物素化技术)表征了flotilin -2在耐洗涤剂膜(DRMs)内的近端相互作用。Flotillin-2在N端或c端与生物素连接酶BirA*融合并在HeLa细胞中表达。通过蔗糖密度梯度超离心分离DRMs,利用生物素亲和和无标记定量(LFQ)质谱法纯化生物素化蛋白。与表达BirA* -mEGFP的对照细胞相比,该方法在表达flotillin-2-BirA *融合的细胞的DRM组分中发现了一组显著富集的蛋白质。这两种分析分别允许在n端和c端BirA*融合中对~ 433和~ 926个独特蛋白进行相对定量。flotilin -2样品中有28 (n端)和88 (c端)蛋白显著富集(28和43的倍数变化≥2)。这些富集蛋白是膜筏结构域内flotilin -2的候选相互作用物。值得注意的是,n端融合相关蛋白与特定的生物过程(如树突运输和信号转导调节)显著相关,而c端融合组显示出膜生物发生相关蛋白的富集。在这里提出的flotilin -2的DRM伙伴中,发现了该蛋白的先前已知和先前未报道的相互作用物。总的来说,我们基于bioid的分析提供了对DRM组分中flotilin -2相互作用组的有价值的见解,并为未来研究探索膜侧异质性的调节和flotilin介导的结构域在信号通路中的作用奠定了基础,特别是在癌症等疾病中失调的信号通路。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Data in Brief
MULTIDISCIPLINARY SCIENCES-
CiteScore
3.10
自引率
0.00%
发文量
996
审稿时长
70 days
期刊介绍:
Data in Brief provides a way for researchers to easily share and reuse each other''s datasets by publishing data articles that: -Thoroughly describe your data, facilitating reproducibility. -Make your data, which is often buried in supplementary material, easier to find. -Increase traffic towards associated research articles and data, leading to more citations. -Open up doors for new collaborations. Because you never know what data will be useful to someone else, Data in Brief welcomes submissions that describe data from all research areas.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


