FATP5 deficiency alleviates MASH via remodeling hepatic lipid composition to suppress ferroptosis
IF 8.2
2区 生物学
Q1 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
Abstract
Metabolic dysfunction-associated steatohepatitis (MASH) represents an advanced stage of fatty liver disease characterized by hepatocyte ballooning, cell death, inflammation and fibrosis. Fatty acid transport protein 5 (FATP5), a hepatocyte-specific transmembrane protein, mediates both long-chain fatty acids (LCFAs) uptake and bile acids (BAs)-coenzyme A (CoA) conjugation. While FATP5 upregulation has been documented in MASH patients, its functional role in disease progression through hepatic lipid metabolic regulation remains unclear. In this study, we identified FATP5 elevation both in vitro and in vivo MASH models. Treatment with the ferroptosis inhibitor ferrostatin-1 (Fer-1) significantly attenuated MASH histopathology and steatotic HepG2 cell death. Furthermore, FATP5 deficiency protected against methionine-choline-deficient diet (MCD)-induced MASH in the mouse model and prevented steatotic HepG2 cell deaths through reducing ferroptosis. Notably, we discovered an inverse correlation between FATP5 and stearoyl-CoA desaturase 1 (SCD1) expression. As the rate-limiting enzyme for monounsaturated fatty acid (MUFA) biosynthesis, SCD1 overexpression exhibited ferroptosis resistance in erastin-treated HepG2 cells. Untargeted lipidomics revealed that FATP5 knockdown preferentially reduced pro-ferroptotic polyunsaturated fatty acid (PUFA)-containing lipids. Mechanistically, reduction of PUFA-lipids alleviated suppression of Sterol regulatory element binding protein 1 (SREBP1), subsequently upregulating its transcriptional target SCD1. Finally, we found that adeno-associated virus-mediated SCD1 overexpression in vivo effectively attenuated inflammation and liver injury in MASH by inhibiting hepatic ferroptosis. In conclusion, our findings suggest that FATP5 knockdown alleviates MASH via remodeling hepatic lipid composition to activate the SREBP1/SCD1 axis, thereby inhibiting ferroptosis. The findings also indicate that FATP5 may serve as a potential therapeutic target of MASH.
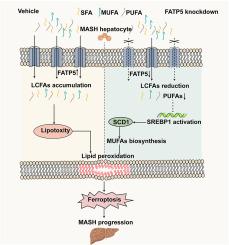
FATP5缺乏通过重塑肝脏脂质组成抑制铁下垂来缓解MASH
代谢功能障碍相关脂肪性肝炎(MASH)是一种晚期脂肪肝疾病,其特征是肝细胞球囊化、细胞死亡、炎症和纤维化。脂肪酸转运蛋白5 (FATP5)是肝细胞特异性跨膜蛋白,介导长链脂肪酸(LCFAs)摄取和胆汁酸(BAs)-辅酶a (CoA)结合。虽然在MASH患者中有FATP5上调的记录,但其通过肝脏脂质代谢调节在疾病进展中的功能作用尚不清楚。在这项研究中,我们在体外和体内的MASH模型中都发现了FATP5的升高。用铁下垂抑制剂铁抑素-1 (fer1)治疗可显著减轻MASH组织病理学和脂肪变性HepG2细胞死亡。此外,在小鼠模型中,FATP5缺乏可以防止蛋氨酸-胆碱缺乏饮食(MCD)诱导的MASH,并通过减少铁下垂来防止脂肪变性HepG2细胞死亡。值得注意的是,我们发现FATP5与硬脂酰辅酶a去饱和酶1 (SCD1)表达呈负相关。作为单不饱和脂肪酸(MUFA)生物合成的限速酶,SCD1过表达在erastin处理的HepG2细胞中表现出铁凋亡抗性。非靶向脂质组学显示,FATP5敲低优先降低了含有亲铁性多不饱和脂肪酸(PUFA)的脂质。机制上,pufa脂质的减少减轻了甾醇调节元件结合蛋白1 (SREBP1)的抑制,随后上调其转录靶点SCD1。最后,我们发现腺相关病毒介导的SCD1在体内过表达通过抑制肝铁下垂有效地减轻了MASH的炎症和肝损伤。总之,我们的研究结果表明,FATP5敲低通过重塑肝脏脂质组成来激活SREBP1/SCD1轴,从而抑制铁下垂,从而减轻MASH。研究结果还表明,FATP5可能是MASH的潜在治疗靶点。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Free Radical Biology and Medicine
医学-内分泌学与代谢
CiteScore
14.00
自引率
4.10%
发文量
850
审稿时长
22 days
期刊介绍:
Free Radical Biology and Medicine is a leading journal in the field of redox biology, which is the study of the role of reactive oxygen species (ROS) and other oxidizing agents in biological systems. The journal serves as a premier forum for publishing innovative and groundbreaking research that explores the redox biology of health and disease, covering a wide range of topics and disciplines. Free Radical Biology and Medicine also commissions Special Issues that highlight recent advances in both basic and clinical research, with a particular emphasis on the mechanisms underlying altered metabolism and redox signaling. These Special Issues aim to provide a focused platform for the latest research in the field, fostering collaboration and knowledge exchange among researchers and clinicians.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


