Epimedokoreanin B blocks autophagic flux to inhibit progression of triple negative breast cancer through targeting MCOLN1/TRPML1 channel
IF 8.2
2区 生物学
Q1 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
Abstract
Triple-negative breast cancer (TNBC) remains a highly malignant subtype with limited therapeutic options. Here, Epimedokoreanin B (EKB), a flavonoid derived from Epimedium brevicornum, is identified as a potent inhibitor of TNBC progression. Mechanistically, EKB directly binds MCOLN1/TRPML1, thereby promoting lysosomal Ca2+ efflux and impairing lysosomal acidification. This disruption specifically blocks autophagosome-lysosome fusion, leading to autophagic flux inhibition and subsequent accumulation of intracellular oxidants, including hydrogen peroxide (H2O2), related peroxides, and mitochondrial superoxide (O2•-). Importantly, the resulting oxidative stress acts as a critical mediator, ultimately triggering apoptosis. The specificity of the EKB-MCOLN1/TRPML1 interaction was validated through molecular docking, Cellular Thermal Shift Assay (CETSA), and pharmacological MCOLN1/TRPML1inhibition. In TNBC mouse models, EKB treatment significantly suppressed primary tumor growth and, notably, reduced pulmonary metastasis. Additionally, no systemic toxicity was observed. Furthermore, EKB treatment reprogrammed the tumor immune microenvironment, evidenced by increased infiltration of CD8+T cells and M1 macrophages alongside reduced immunosuppressive subsets. Taken together, these results establish EKB as a promising therapeutic candidate that couples autophagy blockade with immune activation, and highlight oxidative stress as a significant contributing mechanism underlying its anticancer effects.
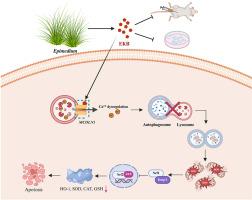
Epimedokoreanin B通过靶向MCOLN1/TRPML1通道阻断自噬通量抑制三阴性乳腺癌进展
三阴性乳腺癌(TNBC)仍然是一种高度恶性的亚型,治疗选择有限。在这里,Epimedokoreanin B (EKB),一种从短角淫羊藿(Epimedium brevicornum)中提取的类黄酮,被认为是TNBC进展的有效抑制剂。在机制上,EKB直接结合MCOLN1/TRPML1,从而促进溶酶体Ca2+外排并损害溶酶体酸化。这种破坏特异性地阻断自噬体与溶酶体的融合,导致自噬通量抑制和随后细胞内氧化剂的积累,包括过氧化氢(H2O2)、相关过氧化物和线粒体超氧化物(O2•-)。重要的是,由此产生的氧化应激是一个关键的介质,最终引发细胞凋亡。EKB-MCOLN1/TRPML1相互作用的特异性通过分子对接、细胞热移测定(CETSA)和药理MCOLN1/TRPML1抑制进行验证。在TNBC小鼠模型中,EKB治疗显著抑制原发肿瘤生长,并显著减少肺转移。此外,未观察到全身毒性。此外,EKB治疗对肿瘤免疫微环境进行了重编程,CD8+T细胞和M1巨噬细胞的浸润增加,免疫抑制亚群减少。综上所述,这些结果表明EKB是一种有希望的治疗候选药物,它将自噬阻断与免疫激活结合起来,并强调氧化应激是其抗癌作用的重要机制。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Free Radical Biology and Medicine
医学-内分泌学与代谢
CiteScore
14.00
自引率
4.10%
发文量
850
审稿时长
22 days
期刊介绍:
Free Radical Biology and Medicine is a leading journal in the field of redox biology, which is the study of the role of reactive oxygen species (ROS) and other oxidizing agents in biological systems. The journal serves as a premier forum for publishing innovative and groundbreaking research that explores the redox biology of health and disease, covering a wide range of topics and disciplines. Free Radical Biology and Medicine also commissions Special Issues that highlight recent advances in both basic and clinical research, with a particular emphasis on the mechanisms underlying altered metabolism and redox signaling. These Special Issues aim to provide a focused platform for the latest research in the field, fostering collaboration and knowledge exchange among researchers and clinicians.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


