DNA methylation signature of oxidative stress and its mediating role in response to metal exposure
IF 8.2
2区 生物学
Q1 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
Abstract
Metal exposure could induce oxidative stress, yet the underlying epigenetic mechanisms remain unclear. Herein, we aimed to investigate the DNA methylation signatures associated with oxidative stress biomarkers, including 8-hydroxyguanine (8-OHdG) and 8-iso-prostaglandin-F2α (8-iso-PGF2α), and evaluate their mediating role in metal-induced oxidative stress. A genome-wide DNA methylation association study was conducted in discovery panel (Shiyan, N = 155) with validation in an independent replication panel (Wuhan-Zhuhai, N = 50). We identified 24 differentially methylated positions (DMPs) associated with 8-OHdG (P < 1 × 10−5), 17 of which were replicated (P < 0.05), along with one validated DMP associated with 8-iso-PGF2α. Two-sample Mendelian randomization analyses supported the causal role of cg15457217 (WDFY1) for 8-OHdG and cg16689883 (LOC284930) for 8-iso-PGF2α. Among the 18 replicated DMPs, five were significantly associated with the expression levels of their annotated genes (KIDINS220, SBF2, DENND1A, PIWIL4, KAT6B). Urinary levels of individual metals (e.g., arsenic, cadmium, molybdenum, strontium) and metal mixtures (assessed by weighted quantile sum regression) were positively associated with both oxidative stress biomarkers. Mediation analyses revealed that 14 replicated DMPs mediated 12.54–33.44 % of the associations for 8-OHdG and 12.60–13.83 % for 8-iso-PGF2α. Notably, cg06197624 (SMIM20) and cg09953898 (KIDINS220) exhibited a significant inverse association with 8-OHdG (FDR < 0.05) and mediated up to 33.44 % of the effect of metal exposure on oxidative DNA damage. These findings demonstrated the mediating role of DNA methylation in metal-induced oxidative stress, highlighting the epigenetic loci involved in redox-related biological responses. Further studies with larger sample sizes and longitudinal assessments are warranted to validate our findings.
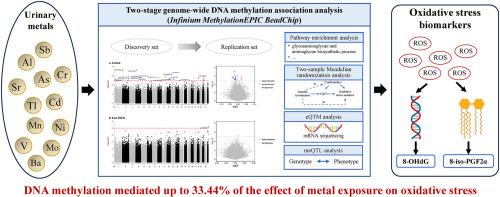
氧化应激的DNA甲基化特征及其在金属暴露反应中的介导作用
金属暴露可诱发氧化应激,但潜在的表观遗传机制尚不清楚。在此,我们旨在研究与氧化应激生物标志物相关的DNA甲基化特征,包括8-羟基鸟嘌呤(8-OHdG)和8-异前列腺素f2 α (8-iso-PGF2α),并评估它们在金属诱导的氧化应激中的介导作用。在发现小组(十堰,N = 155)中进行了全基因组DNA甲基化关联研究,并在独立复制小组(武汉-珠海,N = 50)中进行了验证。我们确定了24个与8-OHdG相关的差异甲基化位点(DMP) (P < 1 × 10−5),其中17个被复制(P < 0.05),以及一个与8-iso-PGF2α相关的验证DMP。双样本孟德尔随机分析支持cg15457217 (WDFY1)与8-OHdG和cg16689883 (LOC284930)与8-iso-PGF2α的因果关系。在18个复制的dmp中,有5个与其注释基因(KIDINS220, SBF2, DENND1A, PIWIL4, KAT6B)的表达水平显著相关。尿液中单个金属(如砷、镉、钼、锶)和金属混合物的水平(通过加权分位数和回归评估)与两种氧化应激生物标志物呈正相关。中介分析显示,14个重复的dmp介导了12.54 - 33.44%的8-OHdG关联和12.60 - 13.83%的8-iso-PGF2α关联。值得注意的是,cg06197624 (SMIM20)和cg09953898 (KIDINS220)与8-OHdG呈显著负相关(FDR < 0.05),介导高达33.44%的金属暴露对DNA氧化损伤的影响。这些发现证明了DNA甲基化在金属诱导的氧化应激中的介导作用,突出了参与氧化还原相关生物反应的表观遗传位点。进一步的研究需要更大的样本量和纵向评估来验证我们的发现。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Free Radical Biology and Medicine
医学-内分泌学与代谢
CiteScore
14.00
自引率
4.10%
发文量
850
审稿时长
22 days
期刊介绍:
Free Radical Biology and Medicine is a leading journal in the field of redox biology, which is the study of the role of reactive oxygen species (ROS) and other oxidizing agents in biological systems. The journal serves as a premier forum for publishing innovative and groundbreaking research that explores the redox biology of health and disease, covering a wide range of topics and disciplines. Free Radical Biology and Medicine also commissions Special Issues that highlight recent advances in both basic and clinical research, with a particular emphasis on the mechanisms underlying altered metabolism and redox signaling. These Special Issues aim to provide a focused platform for the latest research in the field, fostering collaboration and knowledge exchange among researchers and clinicians.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


