Wnt target IQGAP3 promotes Wnt signaling via disrupting Axin1-CK1α interaction
IF 7.3
1区 医学
Q1 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
Abstract
The scaffold protein IQGAP3 is highly upregulated in most epithelial cancers. While recent studies have highlighted its pivotal roles in cancer cell proliferation and metastasis, a deeper mechanistic understanding of IQGAP3 is currently lacking. We have here used TurboID to map IQGAP3 proximity partners and identified the Wnt signaling members Axin1 and CK1α as IQGAP3-interacting proteins. Our functional studies demonstrated that overexpression of IQGAP3 increases β-catenin levels, while IQGAP3 depletion reduces β-catenin levels in gastric cancer cells. Mechanistically, IQGAP3 disrupts Axin1-CK1α interaction, thereby inhibiting β-catenin phosphorylation and ultimately leading to its accumulation. Importantly, we discovered that IQGAP3 itself is regulated by Wnt signaling, suggesting its involvement in a positive feedback loop in Wnt/β-catenin signaling through interactions with Axin1 and CK1α. These findings identify IQGAP3 as a novel mediator of β-catenin stabilization and underscore its potential as a target for cancer therapy.
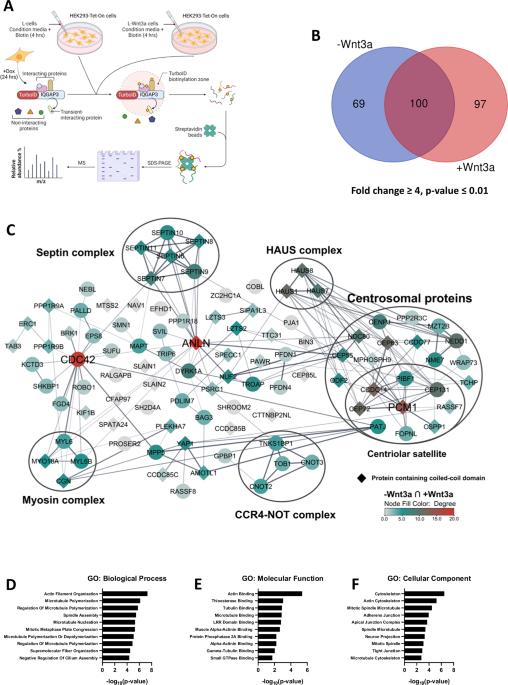
Wnt靶点IQGAP3通过破坏Axin1-CK1α相互作用促进Wnt信号传导。
在大多数上皮癌中,支架蛋白IQGAP3高度上调。虽然最近的研究强调了它在癌细胞增殖和转移中的关键作用,但目前对IQGAP3的机制还缺乏更深入的了解。我们在此使用TurboID绘制了IQGAP3邻近伙伴,并确定了Wnt信号成员Axin1和CK1α是与IQGAP3相互作用的蛋白。我们的功能研究表明,在胃癌细胞中,IQGAP3过表达会增加β-catenin水平,而IQGAP3缺失会降低β-catenin水平。从机制上讲,IQGAP3破坏Axin1-CK1α相互作用,从而抑制β-catenin磷酸化,最终导致其积累。重要的是,我们发现IQGAP3本身受Wnt信号的调控,表明它通过与Axin1和CK1α的相互作用参与Wnt/β-catenin信号的正反馈回路。这些发现确定了IQGAP3是一种新的β-连环蛋白稳定介质,并强调了其作为癌症治疗靶点的潜力。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Oncogene
医学-生化与分子生物学
CiteScore
15.30
自引率
1.20%
发文量
404
审稿时长
1 months
期刊介绍:
Oncogene is dedicated to advancing our understanding of cancer processes through the publication of exceptional research. The journal seeks to disseminate work that challenges conventional theories and contributes to establishing new paradigms in the etio-pathogenesis, diagnosis, treatment, or prevention of cancers. Emphasis is placed on research shedding light on processes driving metastatic spread and providing crucial insights into cancer biology beyond existing knowledge.
Areas covered include the cellular and molecular biology of cancer, resistance to cancer therapies, and the development of improved approaches to enhance survival. Oncogene spans the spectrum of cancer biology, from fundamental and theoretical work to translational, applied, and clinical research, including early and late Phase clinical trials, particularly those with biologic and translational endpoints.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


