Solution-based studies on the contact between the complement receptor 3 ligand-binding domain and simvastatin
IF 2.3
4区 生物学
Q3 BIOCHEMISTRY & MOLECULAR BIOLOGY
Biochimica et biophysica acta. Proteins and proteomics
Pub Date : 2025-08-18
DOI:10.1016/j.bbapap.2025.141096
引用次数: 0
Abstract
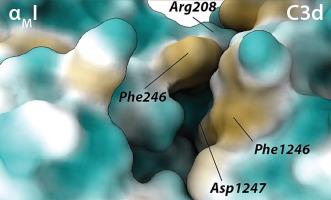
Simvastatin is a primary cholesterol-lowering medication, but it has also been reported to possess anti-inflammatory properties. Notably, the CD18 integrins are targets for simvastatin antagonism of ligand binding, which may affect leukocyte adhesion and diapedesis. Lymphocyte-associated antigen (LFA)-1 is inhibited through an allosteric mechanism by binding the lactone form of simvastatin (simvastatin-lac) to a hydrophobic pocket in the major ligand binding domain, the alpha chain I domain. By contrast, crystallographic evidence showed that complement receptor 3 (CR3) is inhibited by simvastatin in its carboxylate form (simvastatin-carbox) chelated by an Mg2+ ion in the αMI metal ion-dependent adhesion site (MIDAS). We now report that the affinity (KD) of simvastatin-carbox for the αMI is ∼650 μM, which is significantly weaker than the 50 %-inhibitory concentration of simvastatin-lac at 50 μM. The simvastatin-carbox was incapable of inhibiting CR3 binding to iC3b, nor did it exert any neuroprotective or anti-inflammatory effects in the middle cerebral artery occlusion animal model of stroke, unlike what has been reported for simvastatin-lac. From available structural data on the CR3 ligand binding domain in complex with C3d, we suggest that simvastatin-lac makes a critical ternary complex with the ligand binding domain and its ligand before engaging, in its carboxylate form, the MIDAS. In this way, both the LFA-1 and CR3 are antagonized by simvastatin-lac but through fundamentally different mechanisms.
补体受体3配体结合域与辛伐他汀接触的溶液研究
辛伐他汀是一种主要的降胆固醇药物,但也有报道称它具有抗炎特性。值得注意的是,CD18整合素是辛伐他汀拮抗配体结合的靶点,这可能会影响白细胞的粘附和浸润。淋巴细胞相关抗原(LFA)-1通过变构机制被抑制,通过将辛伐他汀(辛伐他汀-lac)的内酯形式结合到主要配体结合域(α链I域)的疏水口袋。相比之下,晶体学证据表明补体受体3 (CR3)被辛伐他汀的羧酸形式(辛伐他汀-carbox)抑制,辛伐他汀在αMI金属离子依赖的粘附位点(MIDAS)被Mg2+离子螯合。我们现在报道辛伐他汀-carbox对α - mi的亲和力(KD)为~ 650 μM,明显弱于辛伐他汀-lac在50 μM时50%的抑制浓度。辛伐他汀-carbox不能抑制CR3与iC3b的结合,也不能在脑卒中大脑中动脉闭塞动物模型中发挥任何神经保护或抗炎作用,这与辛伐他汀-lac的报道不同。根据现有的CR3配体结合域与C3d复合物的结构数据,我们认为辛伐他汀-lac在以羧酸形式与MIDAS结合之前,与配体结合域及其配体形成了一个关键的三元配合物。这样,LFA-1和CR3都被辛伐他汀-lac拮抗,但通过根本不同的机制。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
8.00
自引率
0.00%
发文量
55
审稿时长
33 days
期刊介绍:
BBA Proteins and Proteomics covers protein structure conformation and dynamics; protein folding; protein-ligand interactions; enzyme mechanisms, models and kinetics; protein physical properties and spectroscopy; and proteomics and bioinformatics analyses of protein structure, protein function, or protein regulation.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


