Nitroxoline upregulates low-density lipoprotein receptors expression, enhances lipid metabolism, and reduces hepatic steatosis and atherosclerosis in Apoe−/− mice
IF 4.2
2区 生物学
Q2 BIOCHEMISTRY & MOLECULAR BIOLOGY
Biochimica et biophysica acta. Molecular basis of disease
Pub Date : 2025-08-18
DOI:10.1016/j.bbadis.2025.168016
引用次数: 0
Abstract
Low-density lipoprotein receptors (LDLRs) play a critical role in maintaining cholesterol homeostasis. Dysregulation of lipid metabolism contributes to atherosclerosis and steatohepatitis. This study investigated the effects of nitroxoline on LDLR expression and its protective role in lipid dysregulation, hepatic steatosis, and atherosclerosis. Through comprehensive screening of FDA-approved clinical drugs, nitroxoline was identified as a promising candidate for modulating LDLR. Functional validation in Huh7 cells using quantitative reverse transcription polymerase chain reaction, western blotting, flow cytometry, and RNA-seq analysis showed that nitroxoline significantly upregulated LDLR mRNA and protein expression, enhancing LDL uptake and binding capacity. Mechanistically, nitroxoline promoted SREBF2 expression via PPP2CA suppression and stabilized LDLR mRNA through AMPK- and JNK-dependent repression of the RNA-binding proteins, notably HNRNPD. Transcriptomic profiling revealed increased expression of genes related to cholesterol homeostasis and decreased expression of genes involved in triglyceride biosynthesis, including GPAT, AGPAT, and PNPLA3. In Apoe−/− mice, nitroxoline reduced serum levels of total cholesterol, triglycerides, and LDL-C without affecting HDL![]() C. Histological analyses demonstrated significant reductions in hepatic steatosis, fibrosis, and stellate cell activation, along with a modest attenuation of atherosclerotic plaque formation in the aortic root. These molecular and phenotypic effects were consistent with improved lipid clearance and hepatocellular protection. These findings suggest that nitroxoline exerts dual actions by upregulating LDLR expression and improving hepatic lipid metabolism. Given its established clinical safety and oral bioavailability, nitroxoline may offer repurposing potential as a therapeutic agent for treating lipid-related metabolic disorders and preventing atherosclerotic cardiovascular disease.
C. Histological analyses demonstrated significant reductions in hepatic steatosis, fibrosis, and stellate cell activation, along with a modest attenuation of atherosclerotic plaque formation in the aortic root. These molecular and phenotypic effects were consistent with improved lipid clearance and hepatocellular protection. These findings suggest that nitroxoline exerts dual actions by upregulating LDLR expression and improving hepatic lipid metabolism. Given its established clinical safety and oral bioavailability, nitroxoline may offer repurposing potential as a therapeutic agent for treating lipid-related metabolic disorders and preventing atherosclerotic cardiovascular disease.
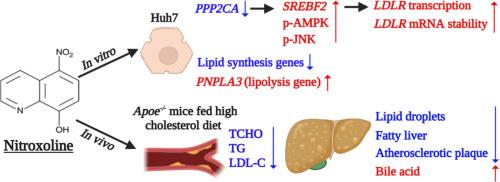
在Apoe - / -小鼠中,硝基喹啉上调低密度脂蛋白受体的表达,增强脂质代谢,减少肝脏脂肪变性和动脉粥样硬化
低密度脂蛋白受体(LDLRs)在维持胆固醇稳态中起着关键作用。脂质代谢失调导致动脉粥样硬化和脂肪性肝炎。本研究探讨了硝基喹啉对LDLR表达的影响及其在脂质失调、肝脂肪变性和动脉粥样硬化中的保护作用。通过对fda批准的临床药物的综合筛选,硝基喹啉被确定为调节LDLR的有希望的候选药物。通过定量逆转录聚合酶链反应、western blotting、流式细胞术和RNA-seq分析对Huh7细胞的功能验证表明,硝基喹啉显著上调LDLR mRNA和蛋白的表达,增强LDL的摄取和结合能力。在机制上,硝基喹啉通过抑制PPP2CA促进SREBF2的表达,并通过AMPK和jnk依赖的rna结合蛋白(特别是HNRNPD)的抑制来稳定LDLR mRNA。转录组学分析显示,与胆固醇稳态相关的基因表达增加,而与甘油三酯生物合成相关的基因表达减少,包括GPAT、AGPAT和PNPLA3。在Apoe−/−小鼠中,硝基喹啉降低了血清总胆固醇、甘油三酯和LDL-C水平,而不影响hdl。组织学分析显示肝脂肪变性、纤维化和星状细胞活化显著减少,同时主动脉根部动脉粥样硬化斑块形成适度减弱。这些分子和表型效应与改善的脂质清除和肝细胞保护一致。上述结果提示,硝基喹啉具有上调LDLR表达和改善肝脏脂质代谢的双重作用。鉴于其已建立的临床安全性和口服生物利用度,硝基喹啉可能作为治疗脂质相关代谢紊乱和预防动脉粥样硬化性心血管疾病的治疗剂提供了重新利用的潜力。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
12.30
自引率
0.00%
发文量
218
审稿时长
32 days
期刊介绍:
BBA Molecular Basis of Disease addresses the biochemistry and molecular genetics of disease processes and models of human disease. This journal covers aspects of aging, cancer, metabolic-, neurological-, and immunological-based disease. Manuscripts focused on using animal models to elucidate biochemical and mechanistic insight in each of these conditions, are particularly encouraged. Manuscripts should emphasize the underlying mechanisms of disease pathways and provide novel contributions to the understanding and/or treatment of these disorders. Highly descriptive and method development submissions may be declined without full review. The submission of uninvited reviews to BBA - Molecular Basis of Disease is strongly discouraged, and any such uninvited review should be accompanied by a coverletter outlining the compelling reasons why the review should be considered.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


