Microbial dysbiosis induced in Cyprinus carpio by tetrabromobisphenol A exposure: Mediation through gut barrier impairment and oxidative stress
IF 2.2
2区 生物学
Q4 BIOCHEMISTRY & MOLECULAR BIOLOGY
Comparative Biochemistry and Physiology D-Genomics & Proteomics
Pub Date : 2025-08-19
DOI:10.1016/j.cbd.2025.101609
引用次数: 0
Abstract
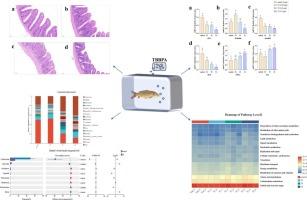
The frequent detection of Tetrabromobisphenol A (TBBPA) in environmental media has elicited considerable scientific concern. The ecotoxicological impacts of TBBPA on intestinal health in the common carp (Cyprinus carpio) were systematically investigated through integrated histopathological, biochemical, and metagenomic analyses. Fish were exposed to environmentally relevant TBBPA concentrations (0, 0.005, 0.05, and 0.5 mg/L) for a 14-day duration. Dose-dependent intestinal damage was induced by TBBPA exposure, manifested as villus fusion and atrophy, oxidative stress (diminished SOD/CAT activities and elevated MDA levels), and downregulated expression of tight junction proteins (ZO-1, Claudin-3, Occludin). This collectively compromised barrier integrity and triggered pro-inflammatory cytokine upregulation of TNF-α alongside anti-inflammatory IL-10 suppression. Significant intestinal microbial dysbiosis was detected via 16S rRNA sequencing. Alpha diversity indices were reduced at low concentrations (0.005 mg/L) of TBBPA but elevated at higher concentrations (0.05–0.5 mg/L). TBBPA exposure induced gut microbiota perturbations, characterized by depletion of beneficial taxa (Cetobacterium) and enrichment of opportunistic pathogens (Legionella and Thermomonas). Functional prediction analyses indicated that these microbial alterations may influence carbohydrate metabolism, and vitamin biosynthesis within the intestinal tract of common carp. Collectively, these findings demonstrated that TBBPA disrupted intestinal health via synergistic mechanisms involving oxidative stress, histopathological damage, and microbiota-mediated dysregulation. This investigation addresses a critical knowledge gap regarding the impacts of TBBPA on fish gut microbiota, while providing provided a reference for assessing the potential ecological risks of TBBPA in the environment.
四溴双酚A暴露诱导鲤鱼微生物生态失调:通过肠道屏障损伤和氧化应激介导
环境介质中频繁检测到四溴双酚A (TBBPA)引起了相当大的科学关注。通过组织病理学、生物化学和宏基因组学综合分析,系统研究了TBBPA对鲤鱼肠道健康的生态毒理学影响。将鱼暴露于与环境相关的TBBPA浓度(0、0.005、0.05和0.5 mg/L)中14天。TBBPA暴露引起剂量依赖性肠损伤,表现为绒毛融合和萎缩,氧化应激(SOD/CAT活性降低,MDA水平升高),紧密连接蛋白(ZO-1, Claudin-3, Occludin)表达下调。这共同破坏了屏障的完整性,并引发了促炎细胞因子TNF-α的上调以及抗炎IL-10的抑制。通过16S rRNA测序检测到明显的肠道微生物失调。α多样性指数在低浓度(0.005 mg/L)下降低,在高浓度(0.05 ~ 0.5 mg/L)下升高。TBBPA暴露诱导肠道微生物群扰动,其特征是有益类群(鲸杆菌)的消耗和机会致病菌(军团菌和热单胞菌)的富集。功能预测分析表明,这些微生物变化可能影响鲤鱼肠道内的碳水化合物代谢和维生素的生物合成。总的来说,这些发现表明TBBPA通过包括氧化应激、组织病理学损伤和微生物群介导的失调在内的协同机制破坏肠道健康。本研究填补了关于TBBPA对鱼类肠道菌群影响的关键知识空白,同时为评估TBBPA在环境中的潜在生态风险提供了参考。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
5.10
自引率
3.30%
发文量
69
审稿时长
33 days
期刊介绍:
Comparative Biochemistry & Physiology (CBP) publishes papers in comparative, environmental and evolutionary physiology.
Part D: Genomics and Proteomics (CBPD), focuses on “omics” approaches to physiology, including comparative and functional genomics, metagenomics, transcriptomics, proteomics, metabolomics, and lipidomics. Most studies employ “omics” and/or system biology to test specific hypotheses about molecular and biochemical mechanisms underlying physiological responses to the environment. We encourage papers that address fundamental questions in comparative physiology and biochemistry rather than studies with a focus that is purely technical, methodological or descriptive in nature.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


