E3 ligase SMURF2 alleviated intrauterine adhesion by stabilizing SMAD6
IF 3.7
2区 生物学
Q1 BIOCHEMISTRY & MOLECULAR BIOLOGY
Biochimica et biophysica acta. Molecular cell research
Pub Date : 2025-08-16
DOI:10.1016/j.bbamcr.2025.120045
引用次数: 0
Abstract
Intrauterine adhesion (IUA) is a debilitating uterine disorder characterized by endometrial fibrosis and infertility, for which effective treatments remain limited. Here, we identify the E3 ubiquitin ligase SMURF2 as a critical protective factor against IUA progression. SMURF2 expression was significantly upregulated in endometrial tissues of IUA patients, a murine IUA model, and TGF-β1-treated human endometrial stromal cells (HESCs). Functional analyses revealed that SMURF2 overexpression mitigated fibrosis-associated phenotypes, including enhanced cell proliferation, migration, and extracellular matrix accumulation, both in vitro and in vivo, whereas SMURF2 knockdown had the opposite effect. Mechanistically, SMURF2 directly interacted with the inhibitory SMAD protein SMAD6 and promoted its stabilization via K63-linked polyubiquitination. Mutation analysis confirmed that disruption of the K63 linkage markedly reduced SMAD6 ubiquitination and destabilized the protein. As a result, SMAD6 accumulation suppressed TGF-β/Smad signaling and downstream fibrotic gene expression. These findings reveal a previously unrecognized SMURF2–SMAD6 axis that counteracts endometrial fibrosis, and suggest that enhancing SMURF2-mediated K63-linked ubiquitination may offer a novel therapeutic avenue for IUA treatment.
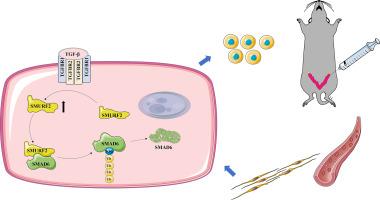
E3连接酶SMURF2通过稳定SMAD6减轻宫内粘连。
宫内粘连(IUA)是一种以子宫内膜纤维化和不孕症为特征的衰弱性子宫疾病,有效的治疗方法仍然有限。在这里,我们发现E3泛素连接酶SMURF2是防止IUA进展的关键保护因子。SMURF2在IUA患者、小鼠IUA模型和TGF-β1处理的人子宫内膜基质细胞(HESCs)的子宫内膜组织中表达显著上调。功能分析显示,SMURF2过表达减轻了纤维化相关表型,包括体外和体内增强的细胞增殖、迁移和细胞外基质积累,而SMURF2敲低则具有相反的效果。在机制上,SMURF2直接与抑制SMAD蛋白SMAD6相互作用,并通过K63-linked多泛素化促进其稳定。突变分析证实,K63连锁的破坏显著降低了SMAD6的泛素化并使该蛋白不稳定。因此,SMAD6的积累抑制了TGF-β/Smad信号传导和下游纤维化基因的表达。这些发现揭示了以前未被识别的SMURF2-SMAD6轴可以抵消子宫内膜纤维化,并表明增强smurf2介导的k63连接泛素化可能为IUA治疗提供新的治疗途径。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
10.00
自引率
2.00%
发文量
151
审稿时长
44 days
期刊介绍:
BBA Molecular Cell Research focuses on understanding the mechanisms of cellular processes at the molecular level. These include aspects of cellular signaling, signal transduction, cell cycle, apoptosis, intracellular trafficking, secretory and endocytic pathways, biogenesis of cell organelles, cytoskeletal structures, cellular interactions, cell/tissue differentiation and cellular enzymology. Also included are studies at the interface between Cell Biology and Biophysics which apply for example novel imaging methods for characterizing cellular processes.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


