Three-Component Cyclization of Phenyl 1,3-Dicarbonyls with α,β-Enaldehydes and 2-(Aminomethyl)aniline to Pyrido[2,1-b]quinazolines
IF 0.9
4区 化学
Q4 CHEMISTRY, ORGANIC
引用次数: 0
Abstract
The three-component condensation of phenyl-substituted 1,3-dicarbonyl compounds, α,β-unsaturated aldehydes, and 2-(aminomethyl)aniline under mild conditions resulted in regio- and chemoselective formation of tetrahydro-7H-pyrido[2,1-b]quinazoline derivatives as mixtures of trans and cis diastereomers, most of which were isolated as individual isomers. Their steric structure was determined by 1H and 13C NMR spectroscopy, including two-dimensional 1H–13C HSQC/HMBC and 1H–1H NOESY experiments, as well as by X-ray analysis.
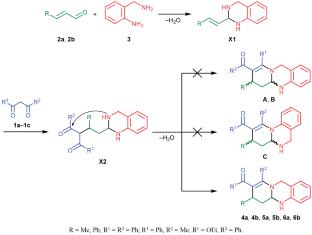

苯基1,3-二羰基与α,β-烯醛和2-(氨基甲基)苯胺的三组分环化合成吡啶[2,1-b]喹唑啉
苯基取代的1,3-二羰基化合物、α、β-不饱和醛和2-(氨基甲基)苯胺在温和条件下的三组分缩合反应,产生了区域选择性和化学选择性的四氢- 7h -吡啶[2,1-b]喹唑啉衍生物,它们是反式和顺式非对映体的混合物,其中大多数是单独的异构体。通过1H和13C核磁共振波谱,包括二维1H - 13C HSQC/HMBC和1H - 1H noesi实验,以及x射线分析确定了它们的位阻结构。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
1.40
自引率
25.00%
发文量
139
审稿时长
3-6 weeks
期刊介绍:
Russian Journal of Organic Chemistry is an international peer reviewed journal that covers all aspects of modern organic chemistry including organic synthesis, theoretical organic chemistry, structure and mechanism, and the application of organometallic compounds in organic synthesis.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


