Performance, Kinetic and Thermodynamic Study of Cobalt (II) Phosphate as a Promising Catalyst for Organic Dyes Removal
Abstract
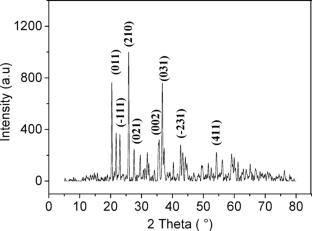
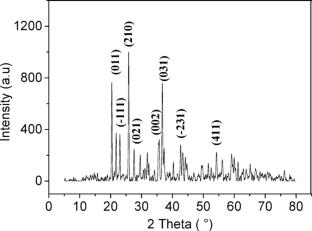
Cobalt phosphate Co3(PO4)2 (CoPO) was synthesized by wet chemical method in the presence of oxalate for its application as a heterogeneous Fenton-like catalyst to remove Basic Yellow 28 (BY-28) dye from water. The formation of the CoPO phase was confirmed by XRD analysis, revealing a crystallite size of 27 nm. The optimal synthesis temperature was found to be 800 °C based on thermal analysis. The modified synthesis method allows for obtaining a homogeneous distribution of spherical grains with surface area of 3.78 m2 g−1. The catalytic activity is attributed to hydroxyl radicals (HO·) generated through the activation of hydrogen peroxide (H2O2). The degradation rate decreased to 10% after 300 min in the presence of 500 mM tert-butyl alcohol (TBA), a well-known HO· scavenger. Several reaction parameters influencing the Fenton-like process were investigated and the highest BY-28 degradation yield was achieved under neutral pH with a CoPO dose of 1.25 g/L and H2O2 concentration of 50 mM. The catalyst was successfully reused five times, maintaining a removal efficiency of 80% after 60 min with a good crystallinity revealed by XRD analysis. Moreover, other organic dyes commonly found in the textile wastewater were also degraded. The kinetic study revealed that the degradation reaction of BY-28 follows the Behnajady-Modirshahla-Ghanbery (BMG) model, while the thermodynamic analysis indicated that the process is non-spontaneous and endothermic. This study offers valuable insights into the performance, kinetics, and thermodynamics of cobalt phosphate-based Fenton-like catalysts for the degradation of organic dyes, highlighting their potential as effective candidates for such applications.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


