Reaction of 5,7-Dihydroxycoumarins with Pyrimidines
IF 0.9
4区 化学
Q4 CHEMISTRY, ORGANIC
引用次数: 0
Abstract
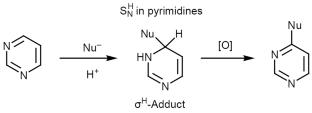
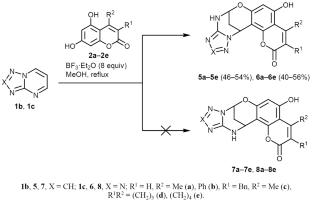
The study of 5,7-dihydroxycoumarins as nucleophilic agents in the SNH reaction with pyrimidines showed that the reaction does not stop at the stage of formation of σH-adduct as intermediate in the SNH mechanism but proceeds further with annulation of the 1,3,5-oxadiazocine ring in moderate to excellent yields. The structures of the obtained compounds were confirmed by NMR spectroscopy and X-ray diffraction. A primary in vitro assessment of their antitumor activity was performed.
5,7-二羟基香豆素与嘧啶的反应
对5,7-二羟基香豆素作为亲核试剂与嘧啶的SNH反应的研究表明,在SNH反应机理中,反应不会止步于生成σ h -加合物作为中间产物的阶段,而会以中至优产率环化1,3,5-恶二唑嘧啶环。所得化合物的结构经核磁共振波谱和x射线衍射证实。对其抗肿瘤活性进行了初步的体外评估。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
1.40
自引率
25.00%
发文量
139
审稿时长
3-6 weeks
期刊介绍:
Russian Journal of Organic Chemistry is an international peer reviewed journal that covers all aspects of modern organic chemistry including organic synthesis, theoretical organic chemistry, structure and mechanism, and the application of organometallic compounds in organic synthesis.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


