Rapid one-step CRISPR-cas vector assembly by isothermal spacer removal linearization and sequence-ligation independent cloning (ISRL-SLIC)
IF 1.9
Q2 MULTIDISCIPLINARY SCIENCES
引用次数: 0
Abstract
CRISPR-Cas genome editing is a powerful tool in various fields, but current cloning methods can be time-consuming due to the frequent use of intermediate entry vectors and multiple steps involving restriction enzymes and ligases. These multiple steps can create a bottleneck in CRISPR-Cas experiments. In response to this challenge, we propose a highly efficient streamlined approach, which enables simultaneous linearization of the acceptor plasmid and protospacer cloning in a single isothermal reaction. This eliminates the need for entry vectors, pre-linearization of vectors, and in vitro ligation, thus significantly simplifying the cloning process. The method can be applied to clone short synthetic oligos for single protospacer constructs or multiple amplicons for multiplex genome editing designs. Either way, researchers can proceed directly to Escherichia coli transformation after a one-hour isothermal reaction and recover the final construct within two days. By combining the advantages of sequence-ligation independent cloning (SLIC) cloning with a streamlined workflow, our approach facilitates rapid and efficient construction of CRISPR-Cas vectors and holds the promise of accelerating research and development in genome editing and related fields.
To expedite the cloning of constructs, we propose a rapid one-step CRISPR-Cas vector assembly method that combines isothermal spacer removal with a sequence-ligation-independent cloning reaction.
We could show that Isothermal Spacer Removal Linearization and Sequence-Ligation Independent Cloning (ISRL-SLIC) can create single, double and triple protospacer constructs in one reaction with scalability.
The ISRL-SLIC reaction delivers clones under a broad range of oligo concentration making it a robust and time saving alternative to other methods for constructing CRISPR-Cas vectors.
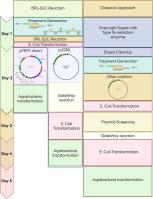
等温间隔段去除线性化和序列连接非依赖性克隆(ISRL-SLIC)快速一步CRISPR-cas载体组装
CRISPR-Cas基因组编辑在许多领域都是一种强大的工具,但目前的克隆方法由于经常使用中间进入载体和涉及限制性内切酶和连接酶的多个步骤而耗时。这些多个步骤可能会在CRISPR-Cas实验中造成瓶颈。为了应对这一挑战,我们提出了一种高效的流线型方法,该方法可以在单个等温反应中同时线性化受体质粒和原间隔物克隆。这消除了进入载体、载体的预线性化和体外结扎的需要,从而大大简化了克隆过程。该方法可用于克隆单个原间隔器构建的短合成寡核苷酸或用于多重基因组编辑设计的多个扩增子。无论哪种方式,研究人员都可以在一小时的等温反应后直接进行大肠杆菌转化,并在两天内恢复最终结构。通过将序列连接独立克隆(SLIC)的优势与简化的工作流程相结合,我们的方法促进了CRISPR-Cas载体的快速高效构建,并有望加速基因组编辑及相关领域的研究和发展。为了加快构建体的克隆,我们提出了一种快速的一步CRISPR-Cas载体组装方法,该方法结合了等温间隔段去除和序列连接无关的克隆反应。我们可以证明等温去除间隔剂线性化和序列连接独立克隆(ISRL-SLIC)可以在一个反应中产生单个、两个和三个原间隔剂结构,并且具有可扩展性。ISRL-SLIC反应在广泛的低聚物浓度下提供克隆,使其成为构建CRISPR-Cas载体的其他方法的可靠且节省时间的替代方法。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

MethodsX
Health Professions-Medical Laboratory Technology
CiteScore
3.60
自引率
5.30%
发文量
314
审稿时长
7 weeks
期刊介绍:
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


