ANAPC5 mitigates acute lung injury through regulating macrophage M1/M2 polarization via the EGFR/CD24 axis
IF 2.9
4区 医学
Q2 CELL BIOLOGY
引用次数: 0
Abstract
Acute lung injury (ALI) is a respiratory disease induced by uncontrolled inflammatory responses in the lungs. The pathological features of ALI include alveolar structural damage and pulmonary edema, which ultimately leads to pulmonary dysfunction. ANAPC5 (Anaphase-promoting complex subunit 5) is an E3 ubiquitin ligase known for its anti-inflammatory properties. This study aims to investigate the effects of ANAPC5 on ALI and its underlying molecular mechanism. In the lung tissue of an ALI mouse model induced by lipopolysaccharide (LPS) administration, we observed downregulation of ANAPC5. Through both in vivo and in vitro experiments, we assessed the effect of ANAPC5 on lung injury by conducting pathological analysis and molecular biological detection. ANAPC5 overexpression alleviated inflammatory cell infiltration, reduced alveolar wall thickening, suppressed pulmonary inflammation, and decreased the levels of inflammatory cytokines in bronchoalveolar lavage fluid (BALF) and lung tissue of the ALI model. Moreover, ANAPC5 inhibited M1 polarization and promoted M2 polarization of macrophages both in vitro and in vivo. We also found that ANAPC5 significantly suppressed the activation and expression of the epidermal growth factor receptor (EGFR) through inducing its ubiquitination in macrophages. In LPS-induced M1 macrophages, the presence of EGFR significantly decreased CD24 expression, followed by reversing the inhibitory effects of ANAPC5 on inflammatory responses and macrophage polarization. Collectively, our findings suggest that ANAPC5 serves as a therapeutic molecular target that mitigates ALI through regulating macrophage M1/M2 polarization via the EGFR/CD24 axis.
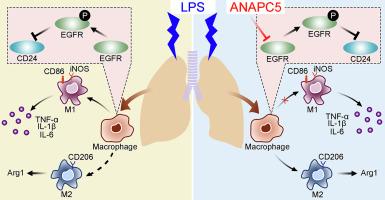
ANAPC5通过EGFR/CD24轴调控巨噬细胞M1/M2极化,减轻急性肺损伤
急性肺损伤(ALI)是一种由肺部不受控制的炎症反应引起的呼吸系统疾病。ALI的病理特征包括肺泡结构损伤和肺水肿,最终导致肺功能障碍。ANAPC5(后期促进复合体亚基5)是一种E3泛素连接酶,以其抗炎特性而闻名。本研究旨在探讨ANAPC5在ALI中的作用及其潜在的分子机制。在脂多糖(LPS)诱导的ALI小鼠模型肺组织中,我们观察到ANAPC5的下调。通过体内和体外实验,我们通过病理分析和分子生物学检测来评估ANAPC5对肺损伤的作用。ANAPC5过表达可减轻ALI模型炎症细胞浸润,减轻肺泡壁增厚,抑制肺部炎症,降低支气管肺泡灌洗液(BALF)和肺组织中炎症因子水平。此外,ANAPC5在体外和体内均能抑制巨噬细胞的M1极化,促进M2极化。我们还发现ANAPC5通过诱导巨噬细胞中表皮生长因子受体(EGFR)的泛素化,显著抑制其激活和表达。在lps诱导的M1巨噬细胞中,EGFR的存在显著降低CD24的表达,随后逆转ANAPC5对炎症反应和巨噬细胞极化的抑制作用。总的来说,我们的研究结果表明ANAPC5作为一种治疗性分子靶点,通过EGFR/CD24轴调节巨噬细胞M1/M2极化来减轻ALI。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Cellular immunology
生物-免疫学
CiteScore
8.20
自引率
2.30%
发文量
102
审稿时长
30 days
期刊介绍:
Cellular Immunology publishes original investigations concerned with the immunological activities of cells in experimental or clinical situations. The scope of the journal encompasses the broad area of in vitro and in vivo studies of cellular immune responses. Purely clinical descriptive studies are not considered.
Research Areas include:
• Antigen receptor sites
• Autoimmunity
• Delayed-type hypersensitivity or cellular immunity
• Immunologic deficiency states and their reconstitution
• Immunologic surveillance and tumor immunity
• Immunomodulation
• Immunotherapy
• Lymphokines and cytokines
• Nonantibody immunity
• Parasite immunology
• Resistance to intracellular microbial and viral infection
• Thymus and lymphocyte immunobiology
• Transplantation immunology
• Tumor immunity.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


